Water-soluble near infrared luminescent quinoline squaraine dye and preparation and application thereof
A water-soluble, quinoline-based technology, applied in the direction of luminescent materials, azo dyes, organic dyes, etc., can solve the problems that the stability needs to be further improved, the application is hindered, and the emission wavelength range is not ideal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
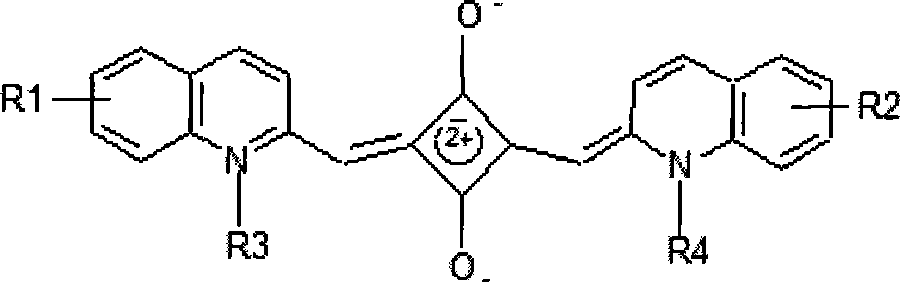
[0091] Synthesis of water-soluble near-infrared luminescent squaraine dyes containing nitro-symmetric quinolines, whose molecular formula is: C 22 h 12 o 6 N 4 R 2 , the structural formula is:
[0092]
[0093] Among them, R=(CH 2 ) n COOH, n=0~7 integers, its properties are brown to dark brown solid, and its melting point is in the range of 330~356°C.
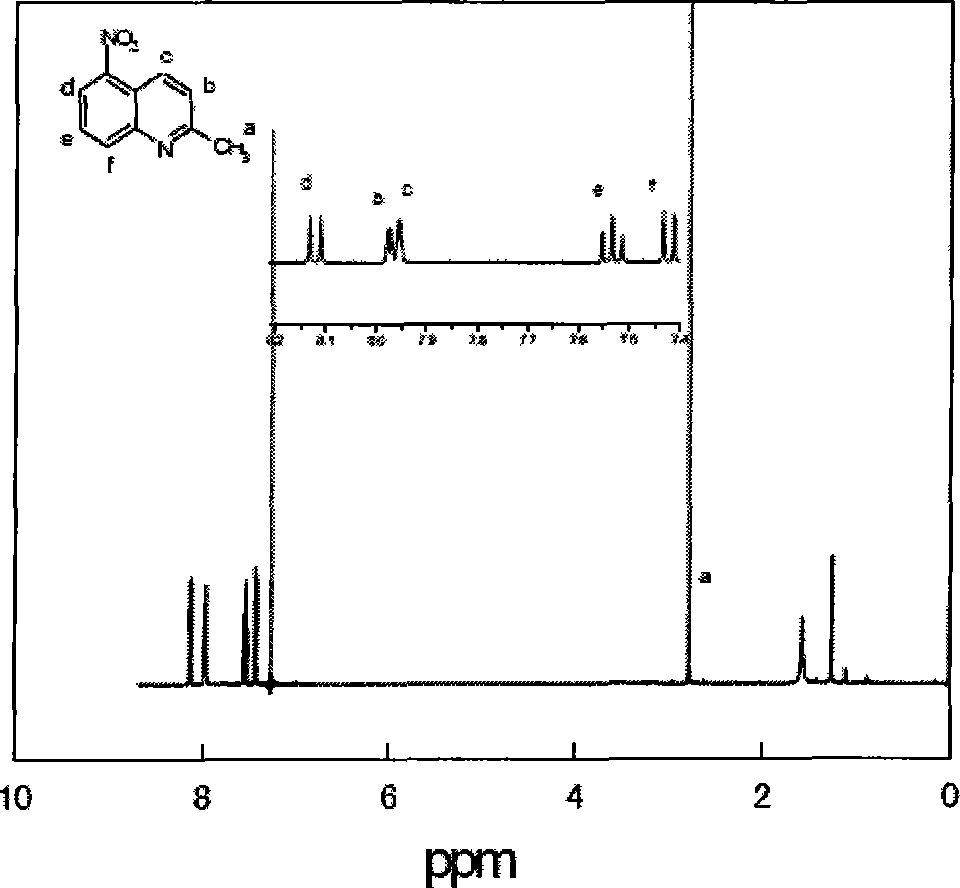
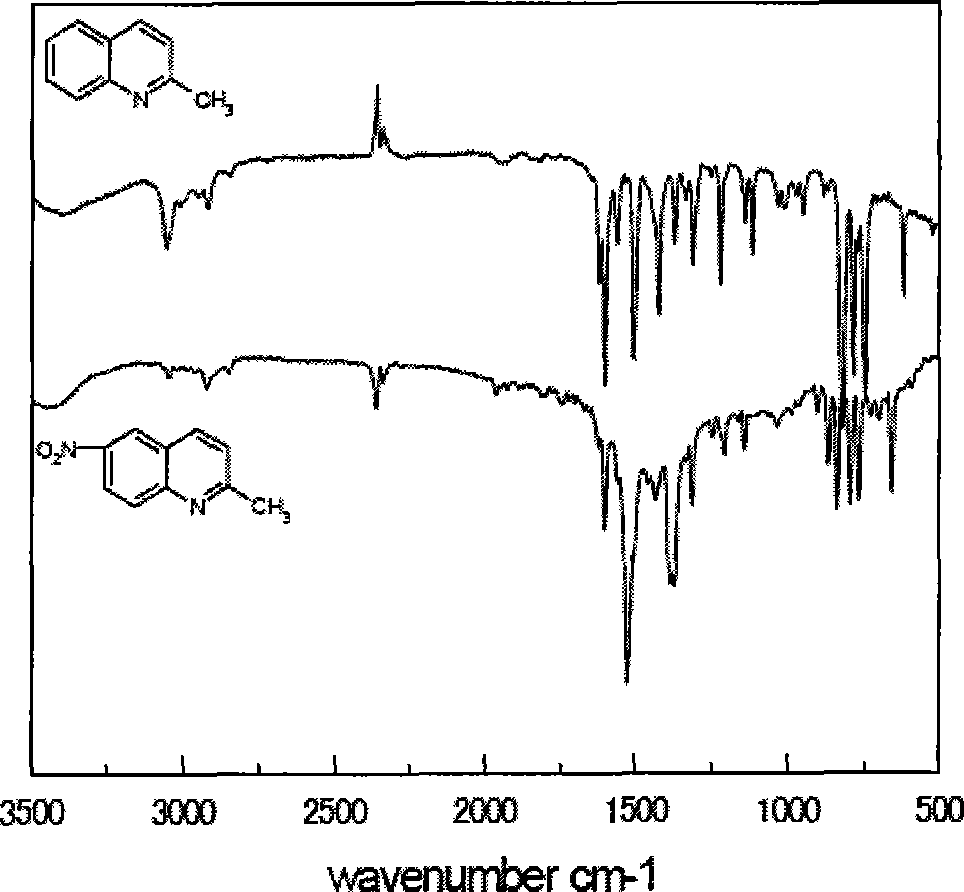
[0094] Add 10mL of concentrated nitric acid and 10mL of concentrated sulfuric acid to a 100mL three-necked flask equipped with a condenser, a thermometer, and a constant pressure dropping funnel under conditions of ice-water bath and magnetic stirring, and slowly add 2mL of 2-methylquinoline dropwise under full stirring to control The reaction temperature is below 10°C. After all the reactants are added, the reaction is continued for 4 hours, and the pH value is adjusted to 3-4 with NaOH solution in an ice-water bath. A large amount of white precipitates are formed. Suction filtration, and then use silica gel as a car...
Embodiment 2
[0101] Synthesis of water-soluble near-infrared luminescent squaraine dyes containing amino-symmetric quinolines. The molecular formula is: C 22 h 16 o 2 N 4 R 2 , the structural formula is:
[0102]
[0103] Among them, R=(CH 2 ) n COOH, n=0~7 integers, the properties are dark gray to brown solid, the melting point is in the range of 293~324°C.
[0104] In a 100mL three-necked flask, add 0.04mol iron powder and 40mL water, then add 2mL glacial acetic acid, boil on low heat for 5min under reflux conditions, cool slightly, add 0.01mol 5 or 6-nitro-2-methylquinoline dropwise , refluxed for 1h under stirring conditions, adding NaCO 3 Adjust to be alkaline, extract the organic phase with dichloromethane, distill under reduced pressure, remove the organic solvent (dichloromethane and a small amount of glacial acetic acid), and then separate by silica gel column chromatography [petroleum ether / ethyl acetate (1:4)] , to obtain the corresponding amino derivatives [5-amino-2...
Embodiment 3
[0108] Synthesis of water-soluble near-infrared luminescent squaraine dyes containing symmetrical quinolines with sulfonic acid groups. The molecular formula is: C 22 h 14 o 8 S 2 N 2 R 2 , the structural formula is:
[0109]
[0110] Among them, R=(CH 2 ) n COOH, n=0~7 integer, the property is black to dark brown solid, the melting point is in the range of 341~365℃.
[0111] A 100mL three-neck flask equipped with a condenser, a thermometer and a constant pressure dropping funnel was placed in an ice-water bath and magnetically stirred, and 20mL of concentrated sulfuric acid was slowly added dropwise with 2mL (0.016mol) of 2-methylquinoline under full stirring to control the reaction. The temperature was below 220°C. After all the reactants were added, the reaction was continued for 3h, and part of the sulfuric acid was neutralized with NaOH in an ice-water bath to make the pH ≈ 6.0, extracted with dichloromethane (20mL×5), the organic phases were combined, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com