Metal-organic framework material for separating xenon and krypton and method for separating xenon and krypton
A technology of metal-organic frameworks and metal ions, applied in separation methods, chemical instruments and methods, and separation of dispersed particles, can solve the problems of easy structure collapse, loss of separation performance, poor stability, etc., and achieve stable performance and good adsorption and separation effects , The effect of stable structural performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
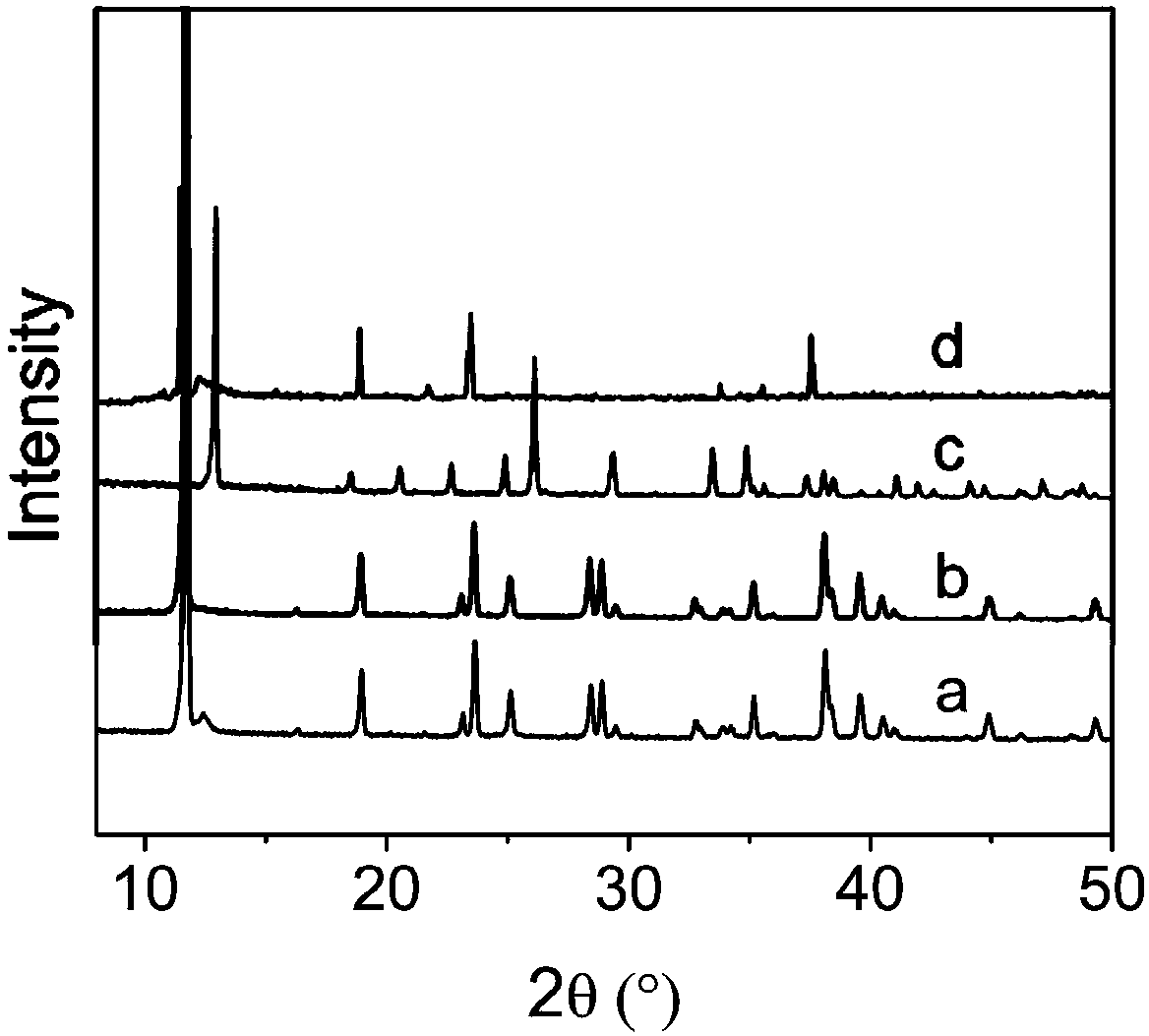
[0045] Mix 1.93mmol cobalt chloride hexahydrate, 2.88mmol squaraine, 7.72mmol potassium hydroxide, and 7ml deionized water, put them into a 25mL hydrothermal reactor, stir for 30 minutes, and then heat to 220°C for 48 hours. After the reaction is completed, the reactor is cooled, and the solid obtained from the reaction is washed with pure water several times to obtain a purified metal-organic framework material. The purified adsorbent was vacuum degassed at 120 °C for 12 hours to obtain the desolvated adsorbent, followed by gas adsorption.
[0046] In order to test the adsorption-separation performance of the above-synthesized MOFs, single-component adsorption isotherms of xenon and krypton were carried out using the above-mentioned adsorbents. Take 100 mg of adsorbent and set the adsorption temperature to 25 degrees. After testing, at 25°C and 1bar, the adsorption capacity of xenon reaches 20.8cm 3 / cm 3 , the adsorption capacity of krypton gas is only 13.1cm 3 / cm 3 , ...
Embodiment 2
[0050] Mix 1.93mmol nickel chloride hexahydrate, 2.88mmol squaraine, 3.86mmol potassium hydroxide, and 7mL deionized water, put them into a 25mL hydrothermal reactor, stir for 30 minutes, and react at 220°C for 48 hours. After the reaction is completed, it is cooled and washed with pure water several times to obtain a purified metal-organic framework material. The purified adsorbent was vacuum degassed at 120 °C for 12 hours to obtain the desolvated adsorbent, followed by gas adsorption.
[0051] In order to test the adsorption-separation performance of the above-synthesized MOFs, single-component adsorption isotherms of xenon and krypton were carried out using the above-mentioned adsorbents. Take 100 mg of adsorbent and set the adsorption temperature to 25 degrees. After testing, at 25°C and 1bar, the adsorption capacity of xenon reaches 35.9cm 3 / cm 3 , the adsorption capacity of krypton gas is only 32.7cm 3 / cm 3 , and at a low pressure of 10kPa, the adsorption capacit...
Embodiment 3
[0055] Mix 0.151mmol calcium carbonate, 0.151mmol squaraine, and 20mL deionized water, put them into a 25mL hydrothermal reaction kettle, stir for 30 minutes, and react at 120°C for 24 hours. After the reaction is completed, the reactor is cooled, and washed with pure water for several times to obtain a purified metal organic framework material. The purified adsorbent was vacuum degassed at 100 °C for 12 h to obtain the desolvated adsorbent, followed by gas adsorption.
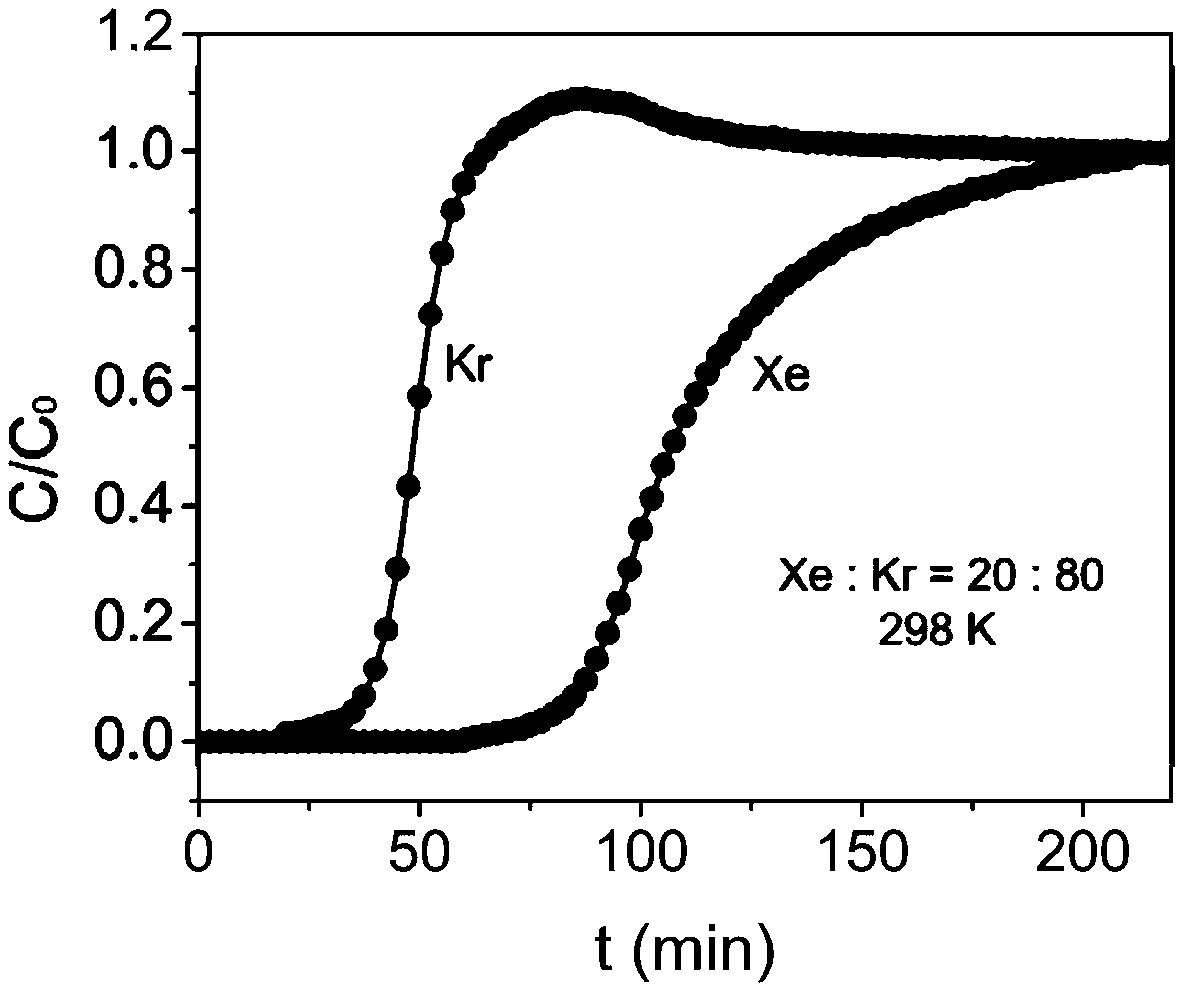
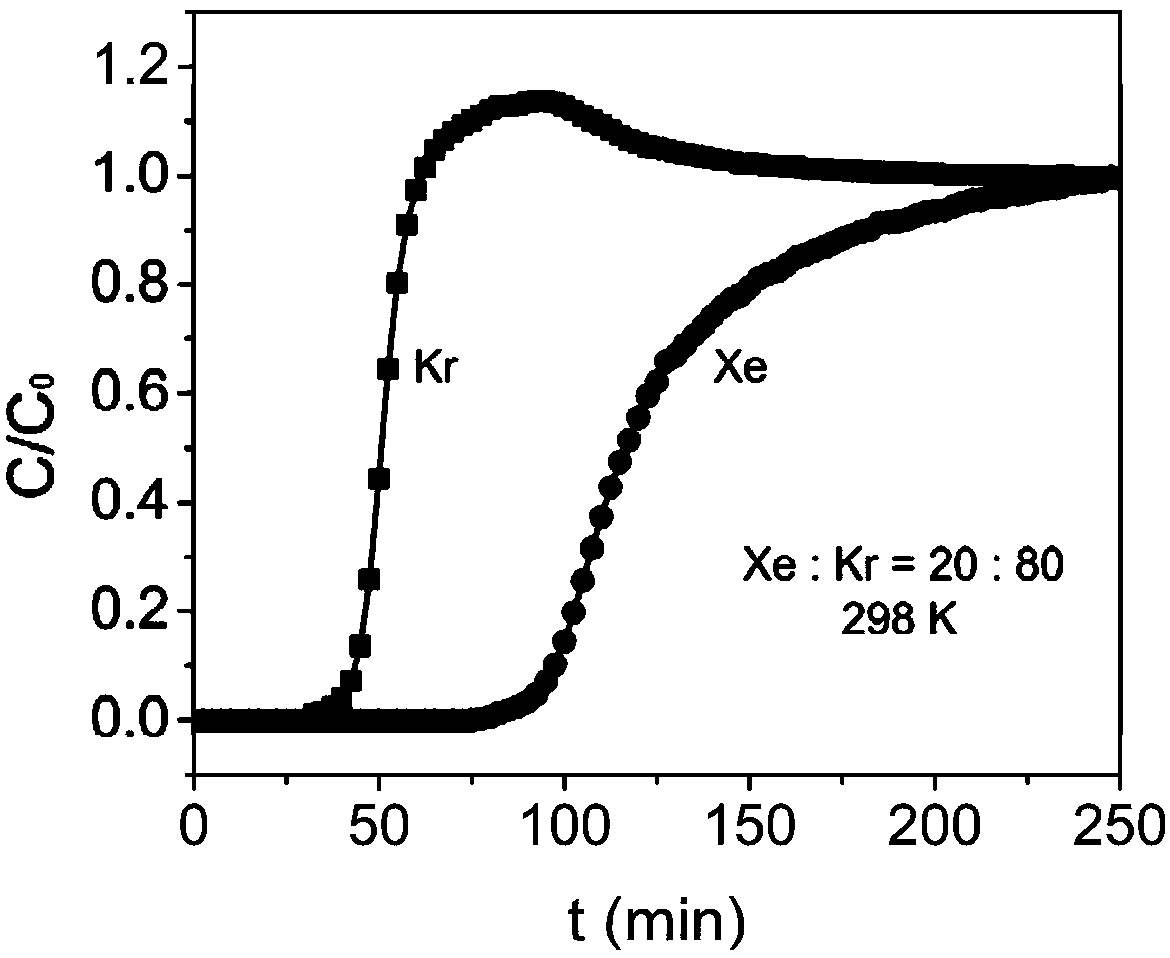
[0056] In order to test the adsorption-separation performance of the above-synthesized MOFs, single-component adsorption isotherms of xenon and krypton were carried out using the above-mentioned adsorbents. Take 100 mg of adsorbent and set the adsorption temperature to 25 degrees. After testing, at 25°C and 1bar, the adsorption capacity of xenon reaches 75.6cm 3 / cm 3 , the adsorption capacity of krypton gas is only 53.3cm 3 / cm 3 , calculated by IAST, when the volume ratio of xenon / krypton is 20:80, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com