Novel indolenium squaraine cyanine dye containing quinazolinone structure
A kind of technology of quinoxalinone and squaraine, applied in the field of novel near-infrared absorption squaraine dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
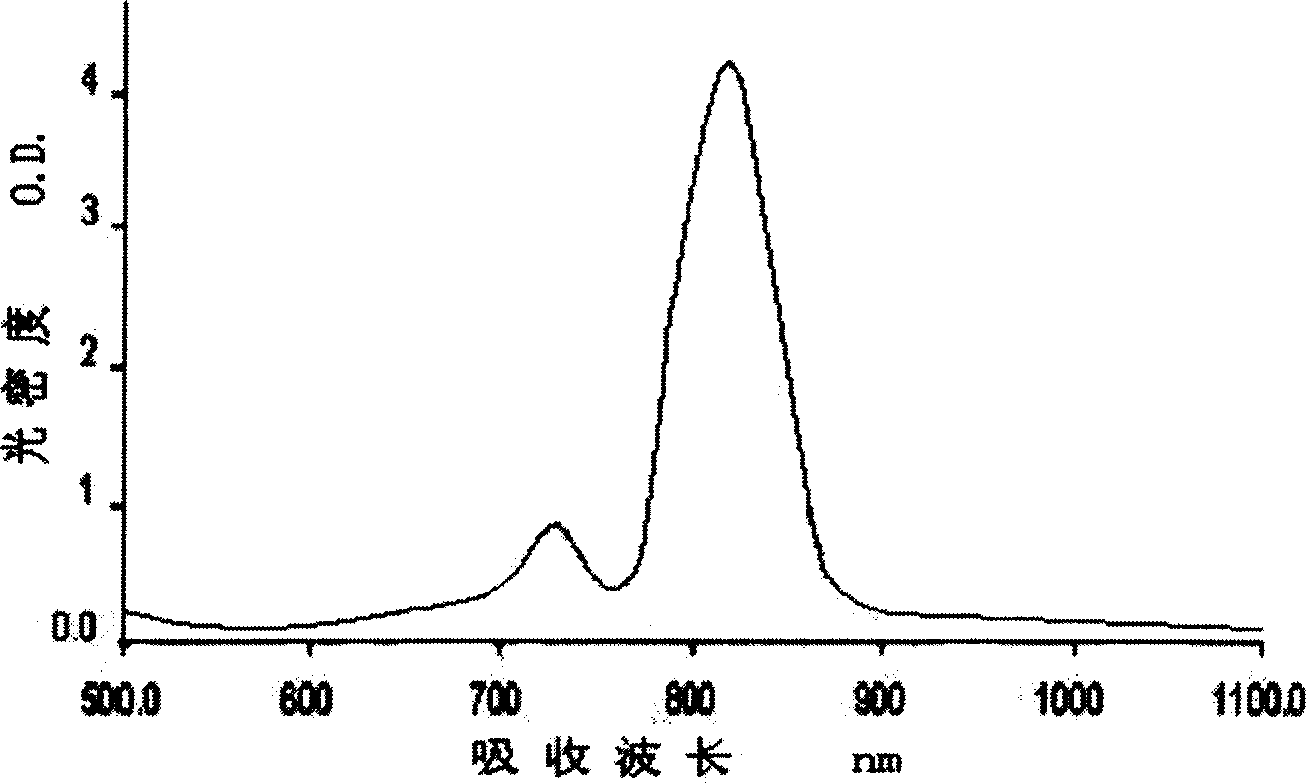
[0069] 3.20g (0.02mol) 3-methyl-2 (1H)-1,4-quinoxalin-2-one and 1.14g (0.01mol) squaraine are placed in the mixed solvent of 20ml n-butanol and 60ml benzene, Stir and reflux for 8 hours under the catalysis of a small amount of pyridine, and the water generated in the reaction is separated with a Dean-Stark water separator. Cool at room temperature, filter, and wash with benzene and petroleum ether successively to obtain a dark green powder product with metallic luster, namely compound [1].
Embodiment 2
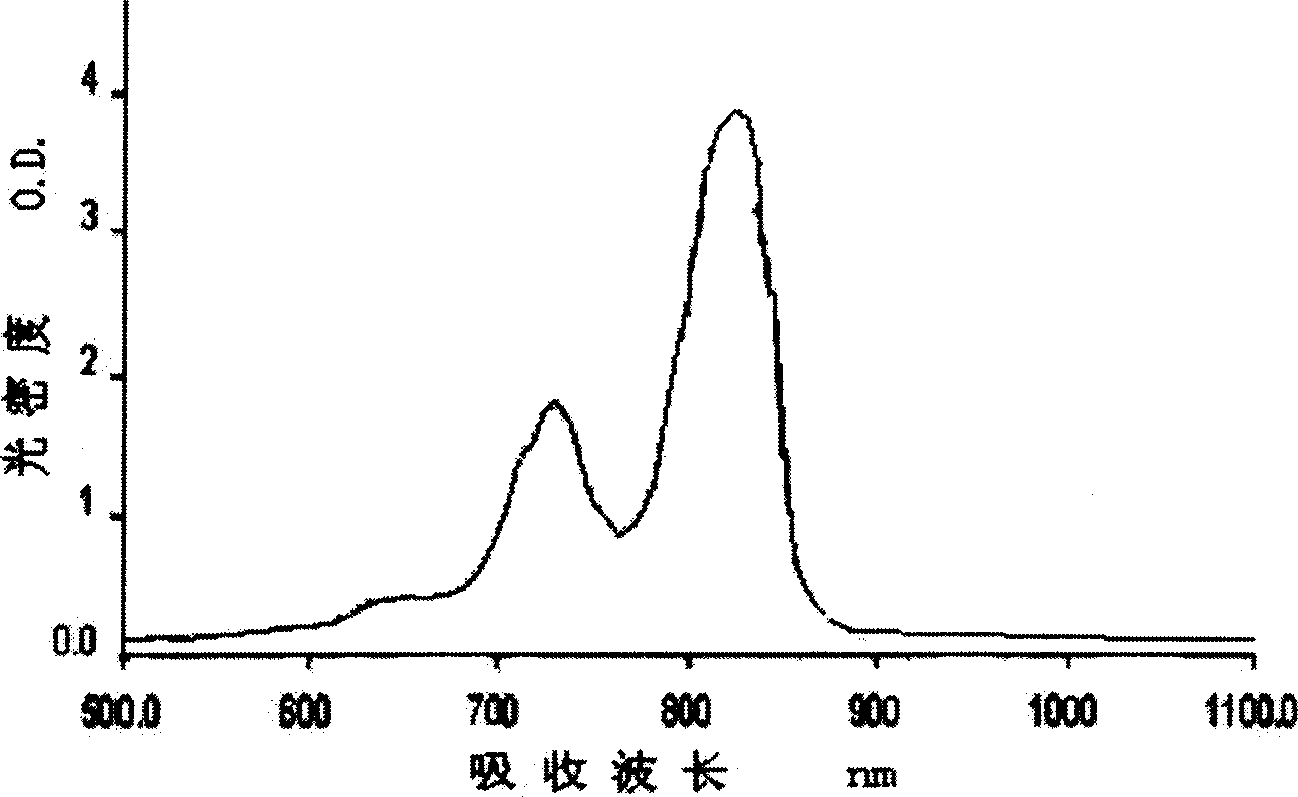
[0071] Put 3.76g (0.02mol) of 1-ethyl-3-methyl-2(1H)-1,4-quinoxalin-2-one and 1.14g (0.01mol) of squaric acid in 40ml of n-propanol and 40ml of toluene In the mixed solvent, stirred and refluxed for 24 h under the catalysis of a small amount of pyridine, and the water generated in the reaction was separated with a Dean-Stark water separator. Cool at room temperature, filter, and wash with benzene, a small amount of ethanol, and petroleum ether successively to obtain a dark green powder product with metallic luster, that is, compound [2].
Embodiment 3
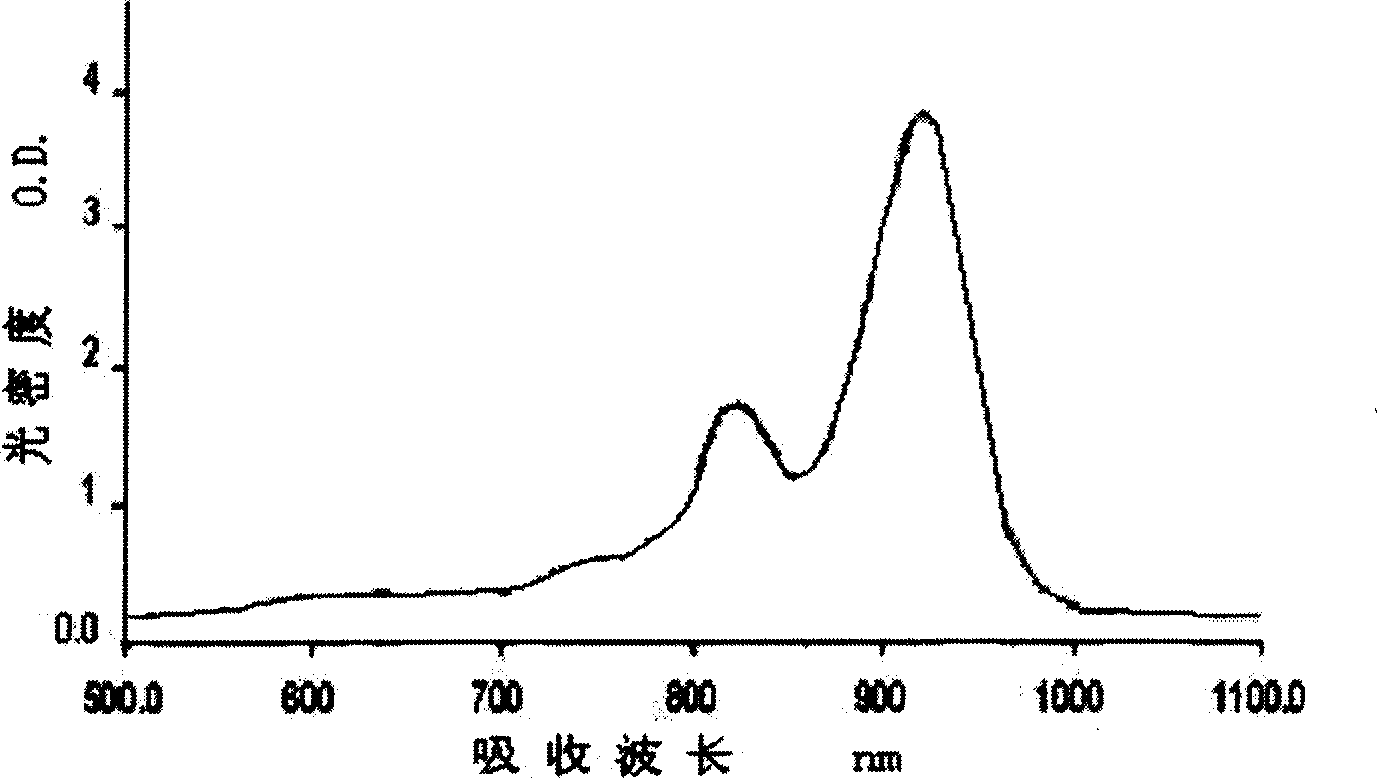
[0073] Put 5.62g (0.02mol) 1-phenyl-3-methyl-6-nitro-2(1H)-1,4-quinoxalin-2-one and 1.14g (0.01mol) squaraine in 40ml In a mixed solvent of isobutanol and 40ml of benzene, stirred and refluxed for 48h under the catalysis of a small amount of quinoline, the water generated in the reaction was separated with a Dean-Stark water separator. Cool at room temperature, filter, and wash with benzene and petroleum ether successively to obtain a dark green product, compound [3].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com