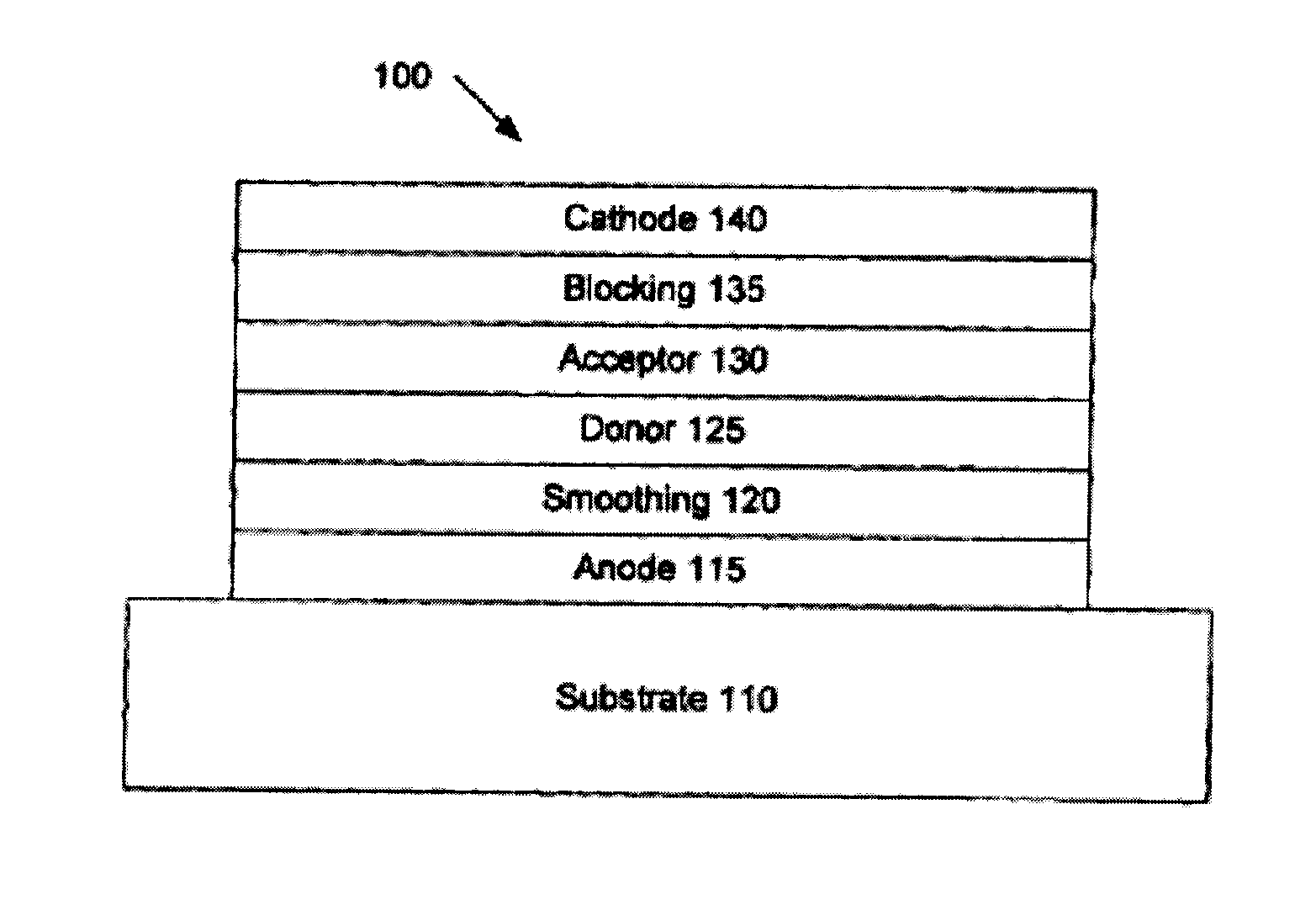
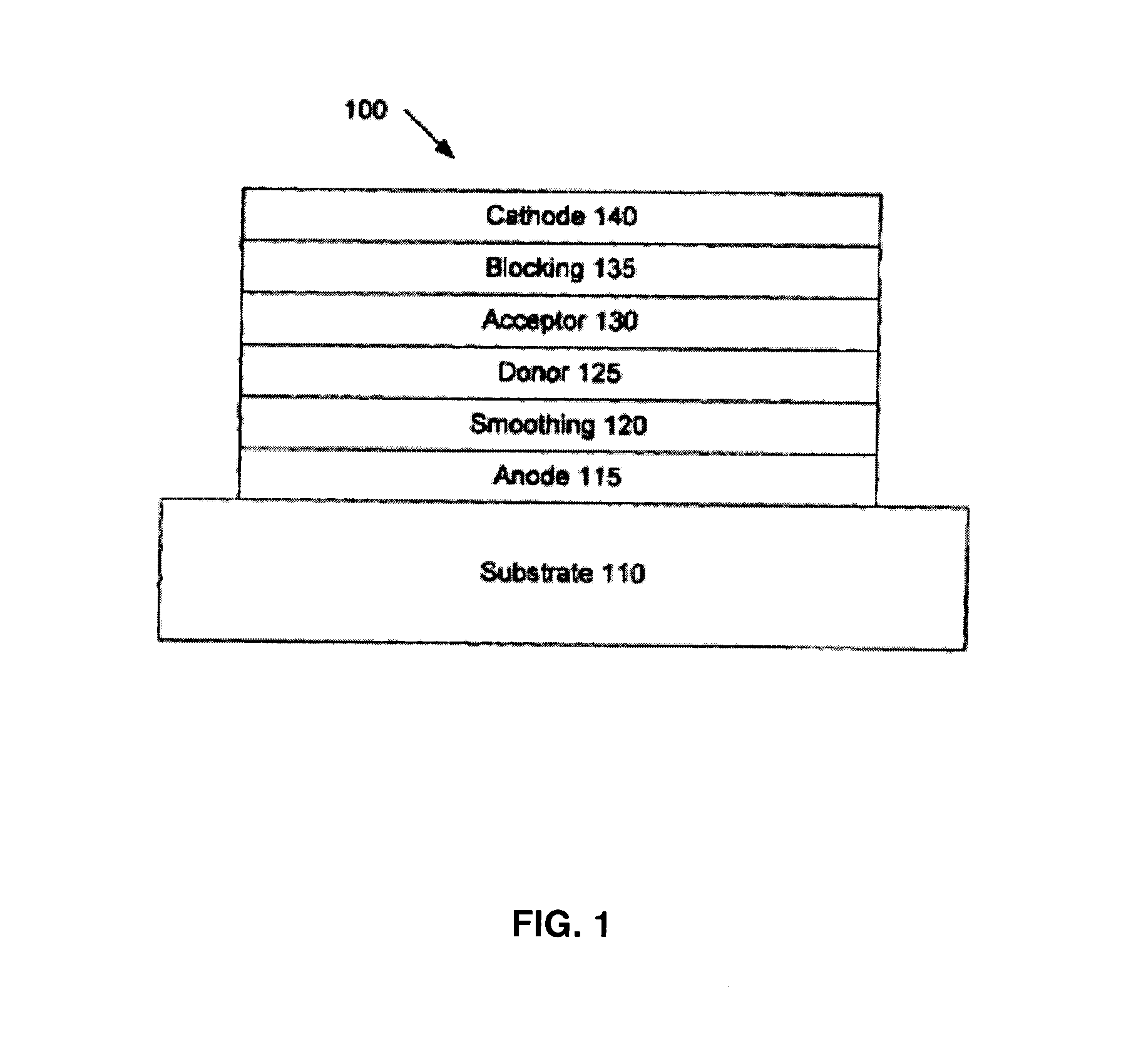
Organic photosensitive devices comprising aryl squaraines and methods of making the same
a technology of organic photosensitive devices and aryl squaraines, which is applied in the field of new squaraine compounds, can solve the problems of difficult and expensive production of efficient crystalline-based devices, low stability of high-efficiency amorphous silicon devices, and inability to provide power to circuits, devices or equipmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CBZSQ: 2,4-bis[4-N-carbazolo-2,6-dihydroxyphenyl] squaraine
[0156]
[0157]1H-NMR (CDCl3, 500 MHz):8.51 (s, 2H), 7.99 (d, 2H), 7.53 (d, 1H), 7.32 (m, 2H), 7.21 (m, 2H), 7.01 (m, 2H), 6.67 (s, 2H)
example 2
DPSQ: 2,4-bis[4-(N,N-diphenylamino)-2,6-dihydroxyphenyl] squaraine
[0158]
[0159]1H-NMR (CDCl3, 500 MHz): 10.1 (s, 1H), 7.41 (t, 2H, J=7.5 Hz), 7.29 (t, 1H, J=5 Hz), 7.23 (d, 2H, J=5 Hz), 5.87 (s, 1H)
[0160]13C-NMR (CDCl3, 500 MHz): 31.29, 50.78, 98.75, 104.96, 127.57, 129.81, 144.08, 159.51, 163.06, 181.36
[0161]MS: m / z 632.2 (MH+).
example 3
1NPSQ:2,4-bis[4-(N-Phenyl-1-naphthylamino)-2,6-dihydroxyphenyl] squaraine
[0162]
[0163]1H-NMR (CDCl3, 400 MHz): 10.90 (s, 2H), 7.81-7.88 (, 3H), 7.44-7.48 (m, 3H), 7.26-7.29 (m, 4H), 5.71 (s, 2H). MS: m / z 732.2 (M+—CH3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Metallic bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com