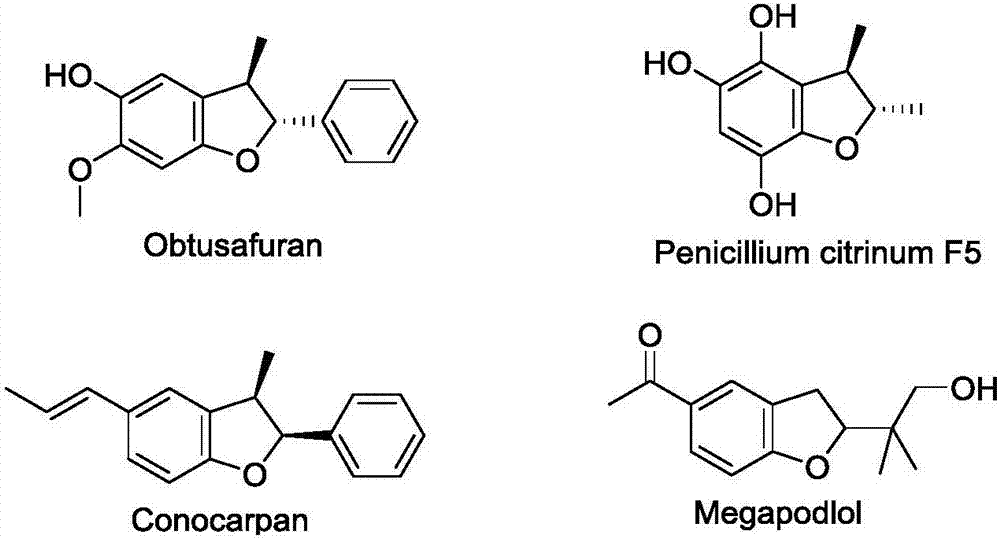
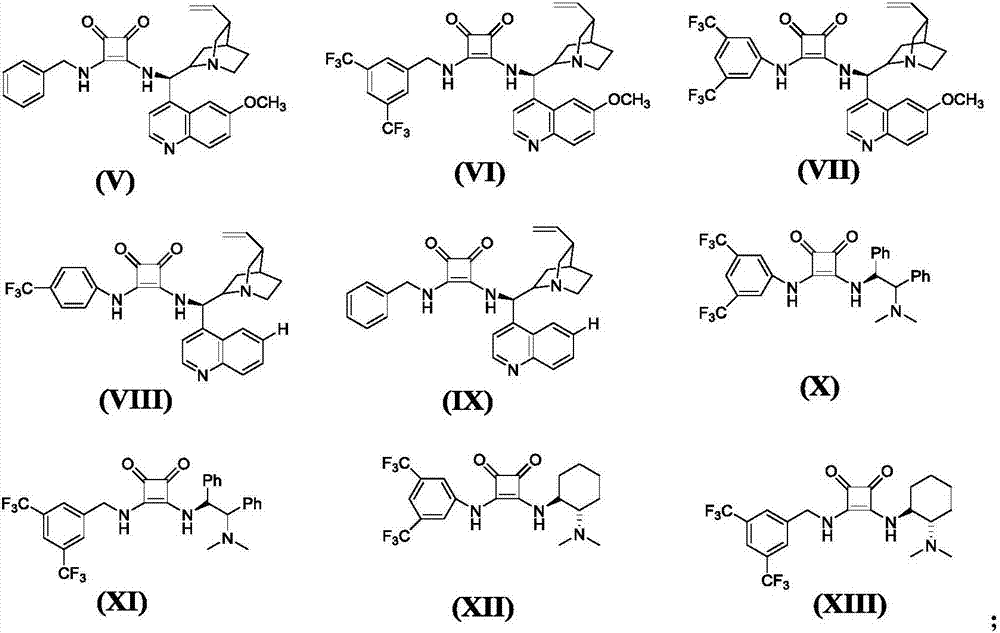
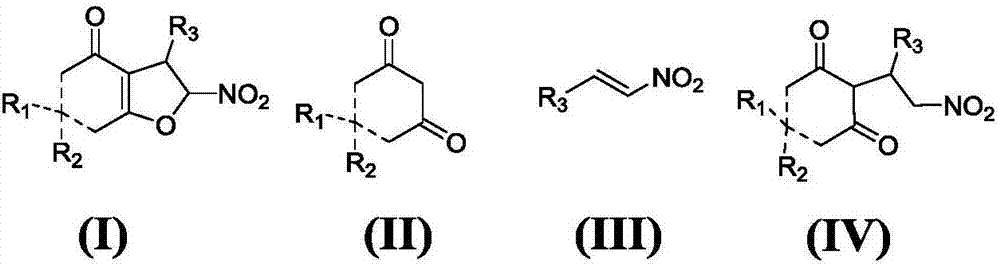
Asymmetric synthesis method for chiral dihydrofuran compound
A technology of dihydrofuran and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as unsatisfactory ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of (2R,3R)-6,6-dimethyl-2-nitro-3-phenyl-3,5,6,7-tetrahydrofuran-4(2H)-one;
[0052]
[0053] (A) Take a 10mL clean small test tube, add 5,5-dimethyl-1,3-cyclohexanedione (0.3mmol, 0.042g), (E)-nitroalkene (0.3mmol, 0.045g), and Squaric acid catalyst V (0.05mmol, 0.00254g), solvent chloroform (2mL). After reacting at 25° C. for 0.5 h, after the complete reaction of the raw materials was detected by TLC, the solvent was distilled off under reduced pressure to obtain a mixture (0.0887 g) containing intermediate compound 1-A.
[0054] (B) In the mixture containing intermediate compound 1-A obtained in the previous step (wherein the amount of 1-A theoretical substance containing 0.3mmol, total mass 0.0887g), add cuprous iodide (0.076g, 0.4mmol), Potassium carbonate (0.6mmol, 0.0682g), solvent acetonitrile (2mL). After reacting at 25°C for 8h, extract with ethyl acetate (3×10mL), and desolvate the organic phase under reduced pressure, use ethyl acet...
Embodiment 2
[0055] Example 2: (2R,3R)-3-(2-anisole)-6,6-dimethyl-2-nitro-3,5,6,7-tetrahydrofuran-4(2H)-one ;
[0056]
[0057] (A) Take a 10mL clean small test tube, add 5,5-dimethyl-1,3-cyclohexanedione (0.3mmol, 0.042g), (E)-2-methoxynitroalkene (0.3mmol, 0.054g), chiral squaraine catalyst VII (0.05mmol, 0.00315g), solvent chloroform (2mL). After reacting at 25°C for 0.5h, after the complete reaction of the raw materials was detected by TLC, the solvent was distilled off under reduced pressure to obtain a mixture containing intermediate compound 2-A (0.094g)
[0058] (B) in the mixture containing intermediate compound 2-A obtained in the previous step (wherein the amount of 2-A theoretical substance containing 0.3mmol, total mass 0.094g), then add iodobenzene acetate (0.4mmol, 0.1288g), Triethylenediamine (0.6mmol, 0.0606g), solvent tetrahydrofuran (3mL), after reacting at 25°C for 6h, extracted with ethyl acetate (3×10mL), the organic phase was desolvated under reduced pressure, a...
Embodiment 3
[0059] Example 3: (2R,3R)-3-(3-anisole)-6,6-dimethyl-2-nitro-3,5,6,7-tetrahydrofuran-4(2H)-one ;
[0060]
[0061] (A) Take a 10mL clean small test tube, add 5,5-dimethyl-1,3-cyclohexanedione (0.3mmol, 0.042g), (E)-3-methoxynitroalkene (0.3mmol, 0.054g), chiral squaraine catalyst IX (0.05mmol, 0.0024g), solvent chloroform (2mL). After reacting at 25° C. for 0.5 h, the raw material was completely reacted by TLC, and the solvent was distilled off under reduced pressure to obtain a mixture (0.094 g) containing intermediate compound 3-A.
[0062] (B) In the mixture containing the intermediate compound 3-A obtained in the previous step (wherein the amount of 3-A theoretical substance is 0.3mmol, and the total mass is 0.094g), add tetrabutylammonium iodide (0.5mmol, 0.1845 g), sodium hydroxide (0.0672g, 0.8mmol), solvent methanol (0.5mL), after 25 ℃ of reaction 4h, extract with ethyl acetate (3 * 10mL), organic phase decompression precipitation, with ethyl acetate: Petroleum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com