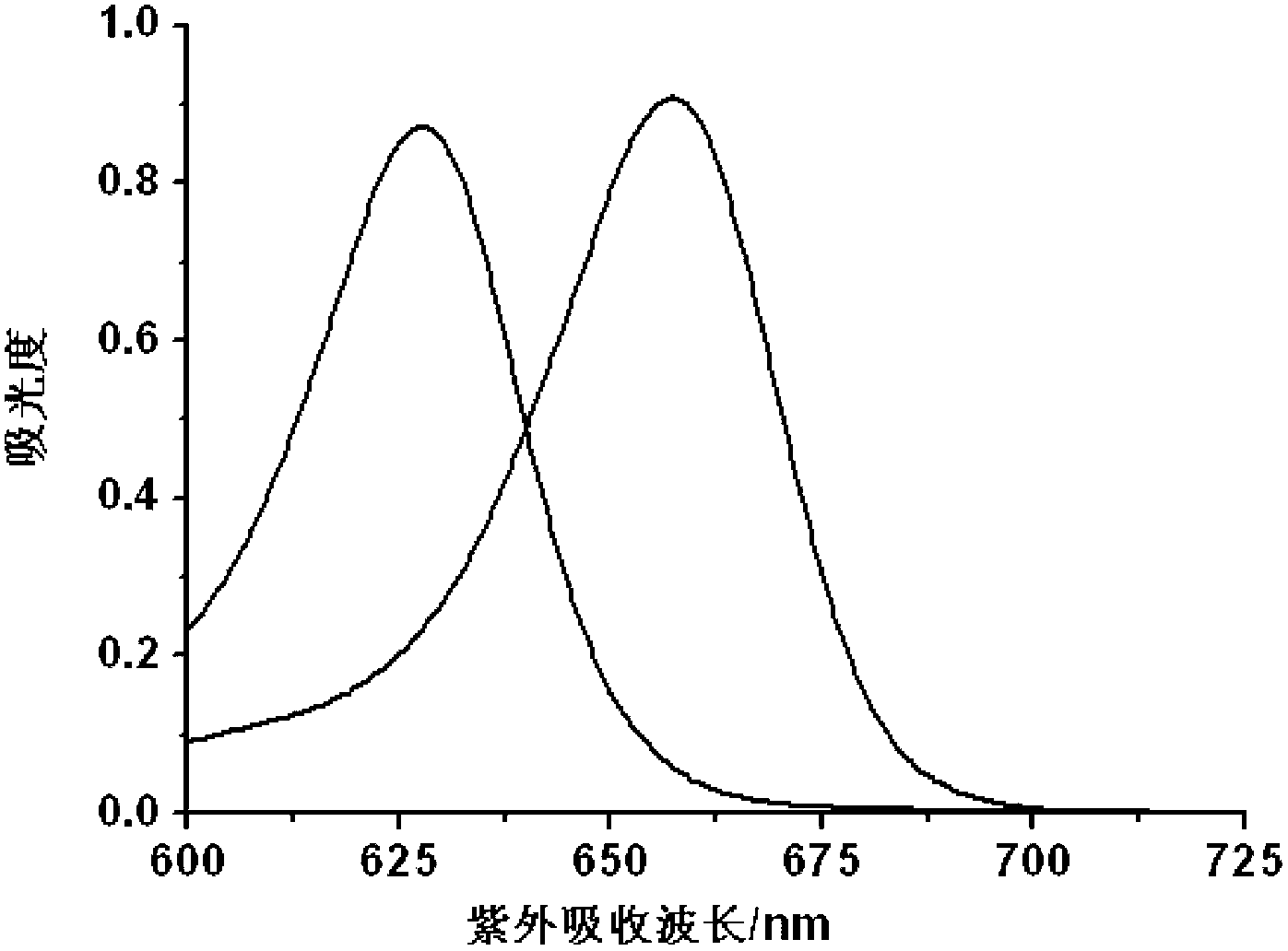
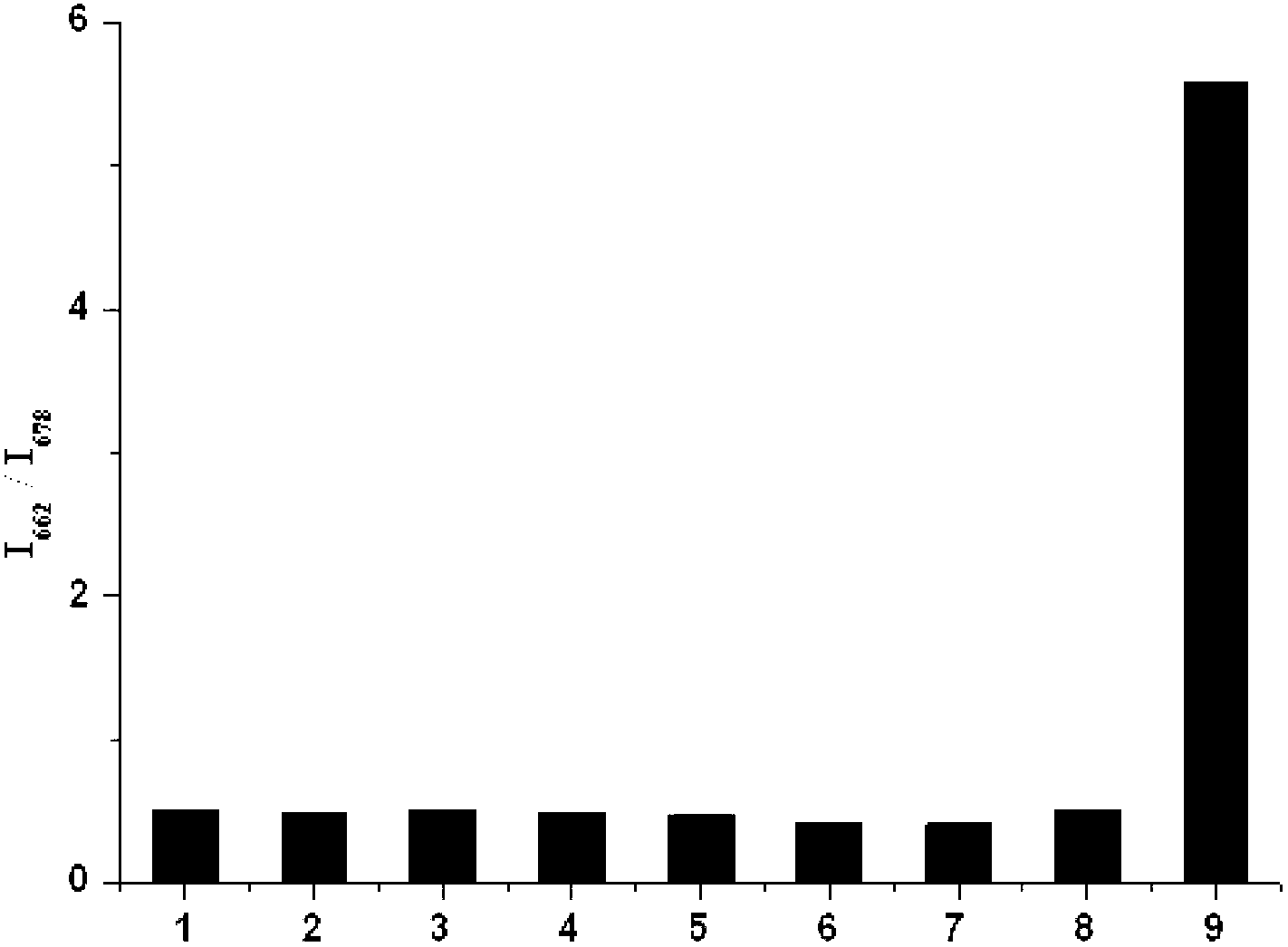
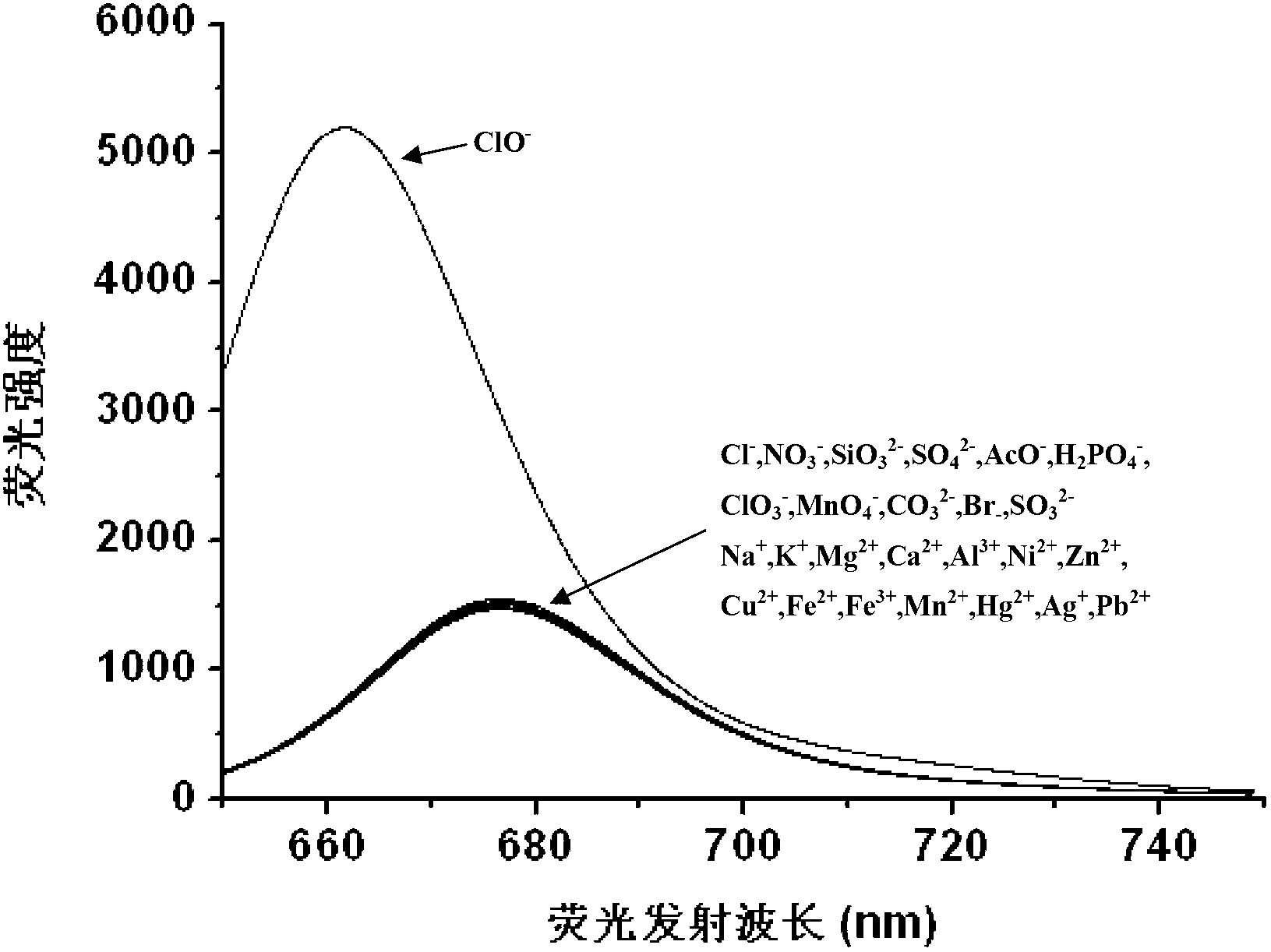
Hypochlorite ion fluorescence probe, and synthetic method and application thereof
A fluorescent probe and hypochlorite technology, applied in the field of chemical analysis, can solve the problems of low hypochlorite content, single action mechanism, and high reactivity, and achieve low detection limit, simple operation process, and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In 20 mL of toluene and n-butanol solvent (1:1 volume ratio), add 3,4-dihydroxy-3-cyclobutene-1,2-dione (1 mmol), 3-N,N-dimethyl Aminophenol (2 mmol) was heated under reflux for 8 hours; after filtration, the precipitate was washed with methanol and dried to obtain 315 mg of intermediate product dimethylamino squaraine dye, with a yield of 81%.
Embodiment 2
[0029] Under the protection of nitrogen, in 20mL of toluene, add the dimethylamino squaraine dye (0.26mmol) and Lawesson (0.4mmol) reagent prepared in step 1), heat to reflux, and react for 10 hours; the solvent was distilled off under reduced pressure, Using silica gel column chromatography (dichloromethane as the mobile phase) to separate and obtain 40 mg of tetramethyl hypochlorite fluorescent probe, the yield is 39%
Embodiment 3
[0031] Add 3,4-dihydroxy-3-cyclobutene-1,2-dione (1.17 mmol), 3-N,N-diethylaminophenol (2.34 mmol) in a molar ratio of 1:2 to 20 mL of toluene and n-butanol (volume ratio: 1:1), stirred and heated to reflux, and reacted for 8 hours; the precipitate was washed three times with methanol, and dried to obtain 0.4088 g of intermediate product, with a yield of 85.5%. 1 H NMR (400MHz, CDCl 3 )δ12.15(s, 1H), 7.94(d, 1H, J=3.2Hz), 7.52(d, 1H, J=2.4Hz), 7.26(d, 1H, J=3.2Hz), 7.00(d, 1H, J=2.4Hz), 6.19(s, 1H), 3.83(s, 1H), 3.50(q, 4H, J=2.4Hz), 1.57(q, 4H, J=2.4Hz), 1.26(t, 6H, J=4.8Hz), 1.10(t, 6H, J=4.8Hz); MS(EI): m / z 408(M + ); HRMS: Cacld.forC 24 h 28 N 2 o 4 [M + ], 408.38; found, 408.391.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com