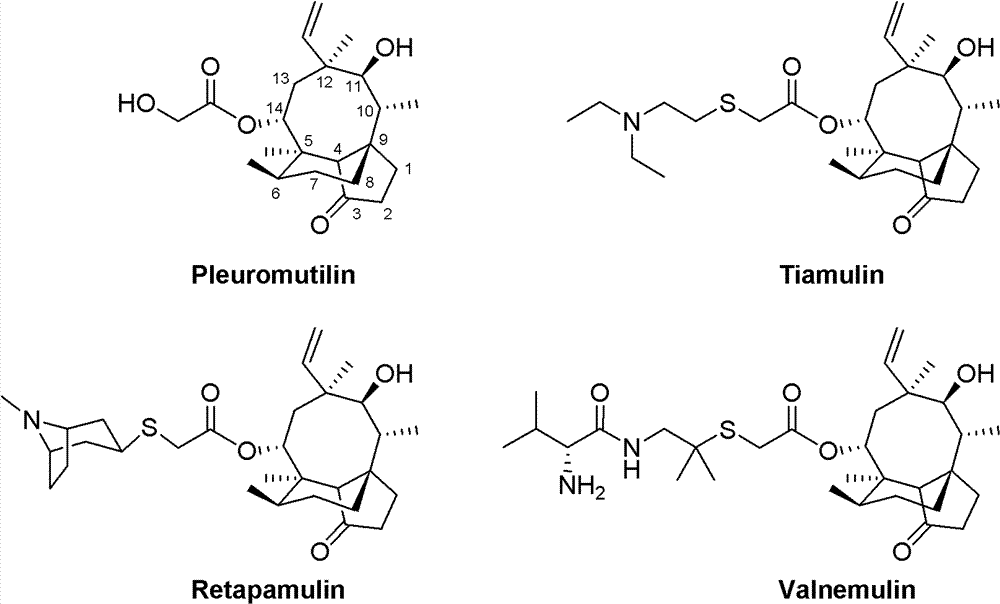
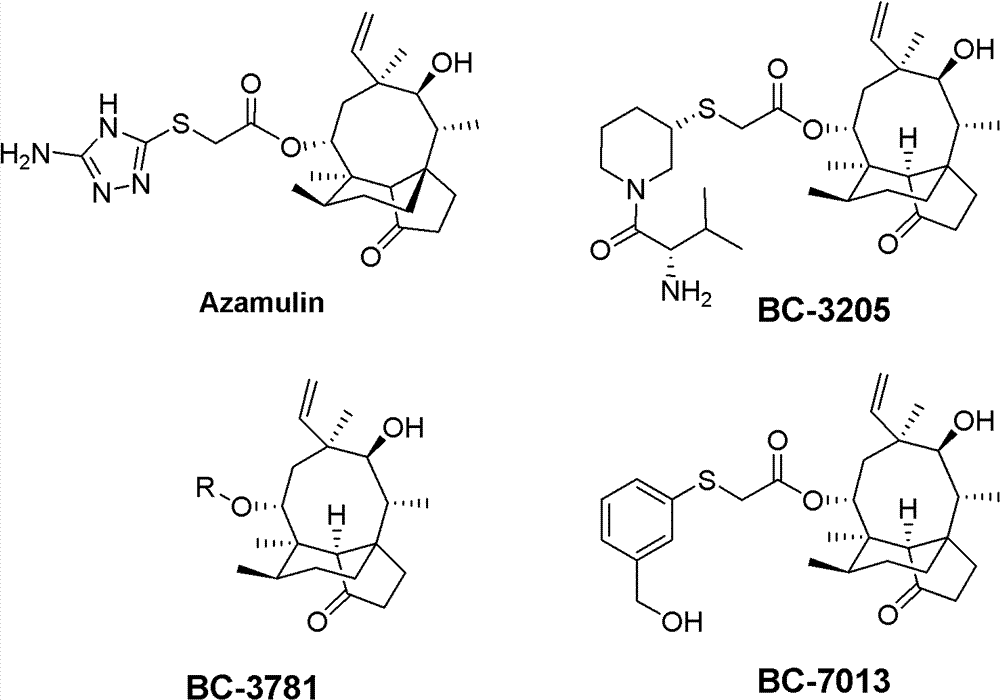
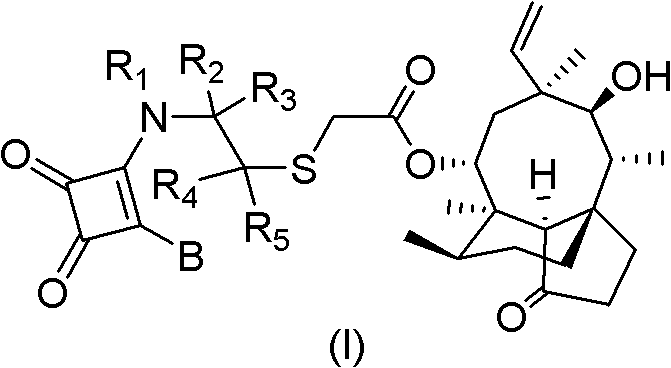
Mulin acetate containing substituted squaric acid and application thereof
A technology of substituents and representatives, which is used in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102]The above-mentioned content of the present invention will be further described in detail through specific implementation in the form of examples below. However, it should not be construed that the scope of the above-mentioned subject matter of the present invention is limited to the following examples. All technologies realized based on the above contents of the present invention belong to the scope of the present invention. Example 1: 2-(1-(2-amino-3,4-dioxocyclobuten-1-yl)amino-2-methylpropyl)thioglycolic acid (3aS, 4R, 5S, 6s, 8R , 9R, 9aR, 10R)-octahydro-5,8-dihydroxy-4,6,9,10-tetramethyl-6-vinyl-3a,9-propane-3aH-cyclopentacyclooctene-1 (4H)-Keto-8-ester is Table Compound 1
[0103] Step 1: Preparation of N-tert-butoxycarbonyl-2-hydroxy-2-methylpropylamine
[0104] 1-Amino-2-methylpropanol (8.9g, 0.1mol) was dissolved in 200ml of dichloromethane, triethylamine (15.2g, 0.15mol) was added, Boc anhydride (26.2g, 0.12mol) was added under stirring at room temperature...
Embodiment 2
[0115] Example 2: 2-(1-(2-(piperazin-1-yl)-3,4-dioxocyclobuten-1-yl)amino-2-methylpropyl)thioglycolic acid (3aS, 4R, 5S, 6S, 8R, 9R, 9aR, 10R)-octahydro-5,8-dihydroxy-4,6,9,10-tetramethyl-6-vinyl-3a,9-propane-3aH- Cyclopentane-1(4H)-one-8-ester is Table Compound 07
[0116] Step 1: Preparation of 3-(piperazin-1-yl)-4-ethoxy-3-cyclobutene-1,2-dione
[0117] Dissolve the diethyl squarylate (1.7 g, 0.01 mol) obtained in Example 1 in 30 ml of absolute ethanol, add piperazine (0.86 g, 0.01 mol), heat to reflux for 12 hours, and concentrate under reduced pressure Add 50 milliliters of ether, stir, precipitate white solid, filter under reduced pressure, obtain 3-(piperazin-1-yl)-4-ethoxyl-3-cyclobutene-1,2-dione (1.49g , 71%). 1 H NMR (400MHz, CDCl 3 )δ4.06(q, 2H), 2.32(t, 4H), 2.18(t, 4H), 1.96(br.s, 1H), 1.21(t, 3H); LC-MS m / z=211[M +H] + .
[0118] Step 2: 2-(1-(2-(piperazin-1-yl)-3,4-dioxocyclobuten-1-yl)amino-2-methylpropan-2-yl)thioglycolic acid (3aS, 4R, 5S, 6S, 8...
Embodiment 3
[0120] Example 3: 2-(1-(2-Amino-3,4-dioxocyclobuten-1-yl)piperidin-3-yl)thioglycolic acid (3aS, 4R, 5S, 6S, 8R, 9R ,9aR,10R)-octahydro-5,8-dihydroxy-4,6,9,10-tetramethyl-6-vinyl-3a,9-propane-3aH-cyclopentacyclooctene-1(4H )-keto-8-ester is the form compound 13
[0121] Step 1: Preparation of N-tert-butoxycarbonyl-3-hydroxypiperidine mesylate
[0122] Dissolve N-tert-butoxycarbonyl-3-hydroxypiperidine (2.01 g, 0.01 mol) in 50 ml of dichloromethane, add 0.02 mol of triethylamine, cool to 0° C. in an ice-water bath, slowly add methanesulfonyl chloride ( 1.37g, 0.012mol), after the dropwise addition, keep stirring at 0°C for 2 hours until the raw material disappears, then add 10ml of saturated ammonium chloride aqueous solution dropwise at 0°C to quench the reaction. The dichloromethane layer was separated by a separating funnel, the aqueous layer was extracted twice with 10 ml of dichloromethane, and the organic layers were combined. The organic phase was washed with 10 ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com