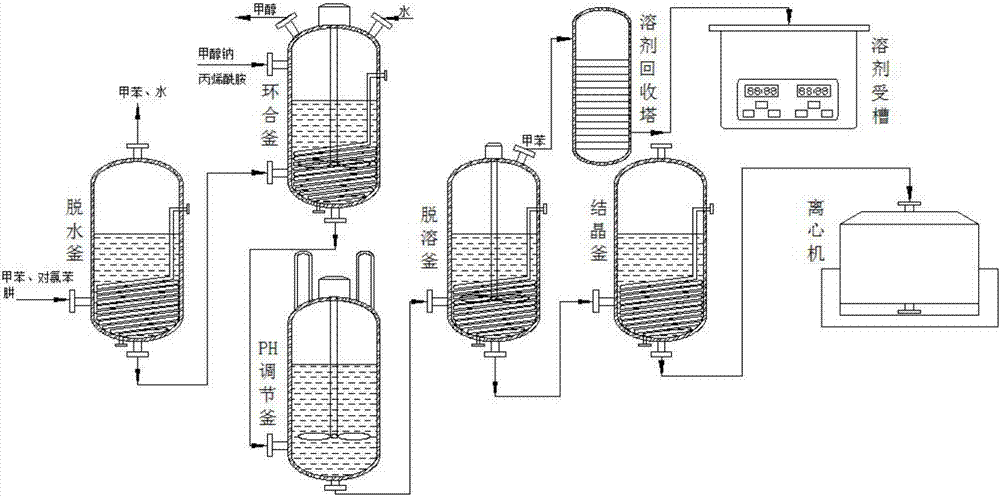
Synthesis process of pyraclostrobin intermediate pyrazolidone
A technology for pyraclostrobin and pyraclostrobin is applied in the field of synthesis technology of pyraclostrobin intermediate pyraclostrobin, which can solve the problem of low yield, waste of production cost, and synthetic efficiency of pyraclostrobin. low problems, to achieve the effect of saving synthesis costs, improving economic benefits, and optimizing synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] see figure 1 As shown, the technical scheme adopted in the present invention is: a synthesis process of pyraclostrobin intermediate pyrazolidinone, and the process method specifically includes the following steps:
[0033] (1). Dehydration: Put the solution containing p-chlorophenylhydrazine and toluene into the dehydration kettle, set the stirring parameters in the dehydration kettle, turn on the mixer for stirring, set the temperature rise parameters and then raise the temperature, and bring the toluene and water to azeotropic dehydration kettle;
[0034] (2). Cycling: Transfer the residue at the bottom of the dehydration kettle to the cycloclosing kettle after the dehydration is completed, and put the sodium methoxide solid into the cyclizing kettle, then add a certain amount of acrylamide to the cyclizing kettle to set After temperature parameters and stirring parameters, heat preservation and stirring are carried out. After the reaction in the ring-closing kettle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com