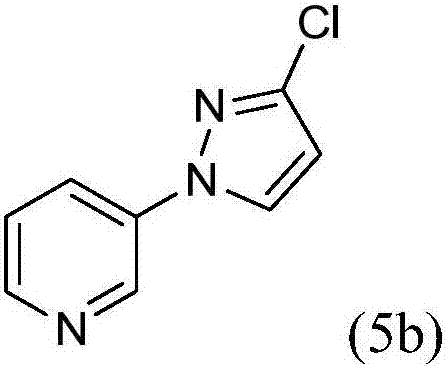
Process for the preparation of 3-(3-chloro-1h-pyrazol-1-yl)pyridine
A technology for pyridine and pyrazole, which is applied in the field of improved preparation of 3-pyridine,-pyrazolidine-3-carboxylate, and can solve problems such as difficult product separation, low yield, and difficult preparation of 3-chloropyrazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
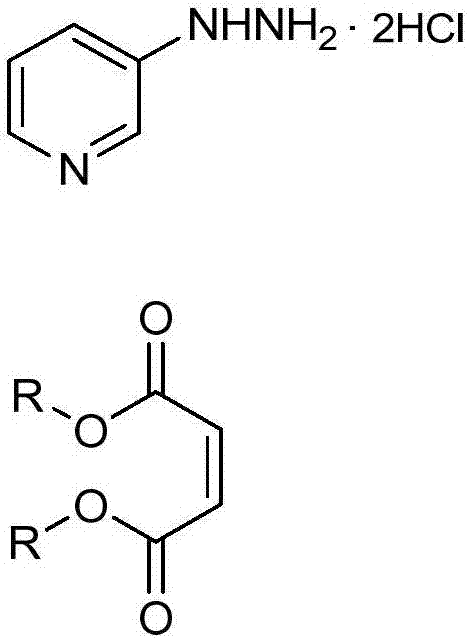
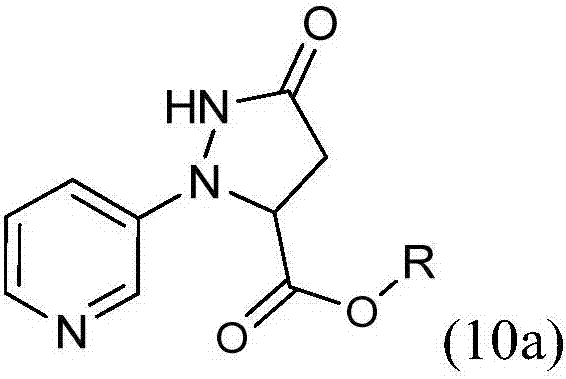
[0025] The present invention provides an improved process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine (5b) by reacting 3-hydrazinopyridine dihydrochloride with dialkyl maleate Cyclization affords alkyl 5-oxo-2-(pyridin-3-yl)pyrazolidine-3-carboxylate (10a), which by chlorination affords 3-chloro-1-(pyridin-3-yl)- 4,5-Dihydro-1H-pyrazole-5-carboxylic acid alkyl ester (10b), by oxidation to give 3-chloro-1-(pyridin-3-yl)-1H-pyrazole-5-carboxylic acid alkyl ester (10c), converted to carboxylic acid by hydrolysis of the ester to give 3-chloro-1-(pyridin-3-yl)-1H-pyrazole-5-carboxylic acid hydrochloride (10d), and removal by decarboxylation carboxylic acid.
[0026] In the first step, the alkali metal (C 1 -C 4 ) alkoxide in the presence of (C 1 -C 4 ) in fatty alcohol with dialkyl maleate to process 3-hydrazinopyridine dihydrochloride to obtain 5-oxo-2-(pyridin-3-yl)pyrazolidine-3-carboxylic acid alkyl ester ( 10a). Although stoichiometric amounts of 3-hydra...
Embodiment
[0038] 1. Preparation of ethyl 5-oxo-2-(pyridin-3-yl)pyrazolidine-3-carboxylate (10a)
[0039]
[0040] A four-neck round bottom flask (250 mL) was charged with sodium ethoxide (21 wt% in ethanol, 56 mL, 192 mmol). 3-Hydrazinopyridine·dihydrochloride (10.0 g, 55.0 mmol) was added, resulting in an exotherm raising the temperature from 20°C to 32°C. The reaction was allowed to cool to 20 °C, diethyl maleate (13.4 mL, 82.0 mmol) was added and the reaction was heated at 60 °C for 3 hours (h). The reaction was cooled to 20°C and quenched with acetic acid. The reaction mixture was diluted with water (100 mL) and extracted with ethyl acetate (3 x 100 mL). The combined organics were concentrated to dryness and the residue was purified by flash column chromatography using ethyl acetate as eluent as the title compound (6.60 g, 51%) as a blue oil: 1 H NMR (400MHz, DMSO-d 6 )δ10.40(s,1H),8.40–8.26(m,1H),8.19(dd,J=4.4,1.6Hz,1H),7.47–7.21(m,2H),4.77(dd,J=9.8, 2.1Hz, 1H), 4.22(qd, ...
Embodiment A
[0061] Example A Bioassays on Green Peach Aphid ("GPA") (Myzus persicae) (MYZUPE).
[0062] GPA is the most important aphid pest of peach trees, causing reduced growth, shriveling of leaves and death of various tissues. It is also dangerous because it acts as a vector for the transport of plant viruses, such as potato virus Y and potato leafroll virus (for members of the nightshade / potato family Solanaceae), and various mosaic viruses (for many other grain crop). GPA attacks plants such as broccoli, burdock, cabbage, carrots, cauliflower, daikon, eggplant, kidney beans, lettuce, macadamia nuts, papaya, peppers, sweet potatoes, tomatoes, watercress, and zucchini, among others. GPA also attacks many ornamental crops such as carnations, chrysanthemums, flowering white cabbage, orangutan and roses. GPA has developed resistance to many insecticides.
[0063] Several molecules disclosed herein were tested for GPA using the procedure described below.
[0064] Cabbage seedlings (w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com