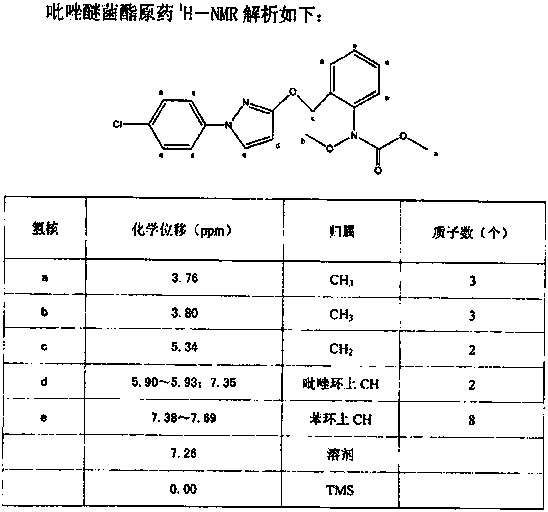
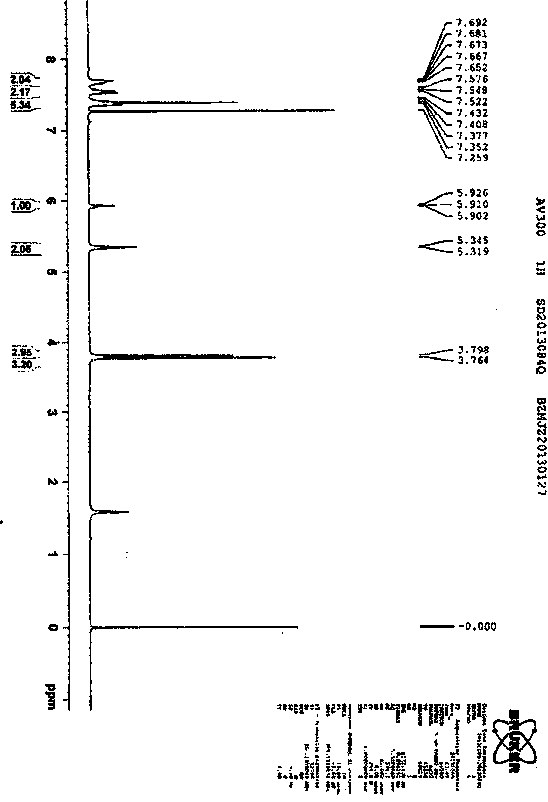
Synthetic technology for pyraclostrobin
A technology of pyraclostrobin and synthesis process, which is applied in the field of preparation of original drug compounds, can solve the problems of difficult control of hydroxylamine, long reaction route, difficult purification of reactants, etc., and achieve the effect of easy control and stable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Synthesis of 1-(4-chlorophenyl)-pyrazolidin-3-one
[0050] .
[0051] In a four-necked reaction flask equipped with a stirrer, a thermometer, a reflux condenser and a dropping funnel, add 80ml of absolute ethanol and 1.38g (0.06mol) of sodium metal, cool to room temperature, and add 14.25g (0.1mol) of 4-Chlorophenylhydrazine, 12g (0.12mol) ethyl acrylate was added dropwise, after the dropwise addition was completed, it was raised to reflux, stirred for 6h, and the reaction mixture was poured into ice water, a large amount of white solids were precipitated, filtered and dried to obtain 18.4g solids, collected The rate is 92.1%.
[0052] 2. Synthesis of 1-(4-chlorophenyl)-3-pyrazolol
[0053] .
[0054] Add 19.6g (0.1mol) 1-(4-chlorophenyl)pyrazolidin-3-one and 100ml acetonitrile into a 250ml four-necked reaction flask with mechanical stirring, reflux condenser and thermometer, stir to dissolve, then add 2g (0.02mol) of concentrated sulfuric acid and 32.4g (0.1...
Embodiment 2
[0068] 1. Synthesis of 1-(4-chlorophenyl)-pyrazolidin-3-one
[0069] .
[0070] In a four-necked reaction flask equipped with a stirrer, a thermometer, a reflux condenser and a dropping funnel, add 80ml of absolute ethanol and 1.38g (0.06mol) of sodium metal, cool to room temperature, and add 14.25g (0.1mol) of 4-Chlorophenylhydrazine, add 12g (0.12mol) ethyl acrylate dropwise, after the dropwise addition is completed, rise to reflux, stir for 6h, pour the reaction mixture into ice water, a large amount of white solid precipitates, filter and dry to obtain 18.1g solid, collect The rate is 91.9%.
[0071] 2. Synthesis of 1-(4-chlorophenyl)-3-pyrazolol
[0072] .
[0073] Add 0.1mol1-(4-chlorophenyl)pyrazolidin-3-one and 100ml tetrahydrofuran in a 250ml four-necked reaction flask with mechanical stirring, reflux condenser and thermometer, after stirring and dissolving, add 0.02mol concentrated sulfuric acid and 0.2 mol potassium persulfate, heated to reflux at 30-80°C (o...
Embodiment 3
[0087] 1. Synthesis of 1-(4-chlorophenyl)-pyrazolidin-3-one
[0088] .
[0089] In a four-necked reaction flask equipped with a stirrer, a thermometer, a reflux condenser and a dropping funnel, add 80ml of absolute ethanol and 1.38g (0.06mol) of sodium metal, cool to room temperature, and add 14.25g (0.1mol) of 4-Chlorophenylhydrazine, 12g (0.12mol) ethyl acrylate was added dropwise, after the dropwise addition was completed, it was raised to reflux, stirred for 6h, the reaction mixture was poured into ice water, a large amount of white solid precipitated, filtered and dried to obtain 18.6g solid, collected The rate is 92.3%.
[0090] 2. Synthesis of 1-(4-chlorophenyl)-3-pyrazolol
[0091] .
[0092] Add 0.1mol 1-(4-chlorophenyl)pyrazolidin-3-one and 100ml ethanol or methanol into a 250ml four-necked reaction flask with mechanical stirring, reflux condenser and thermometer, stir to dissolve, then add 0.02mol concentrated Sulfuric acid and 0.1mol potassium persulfate we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com