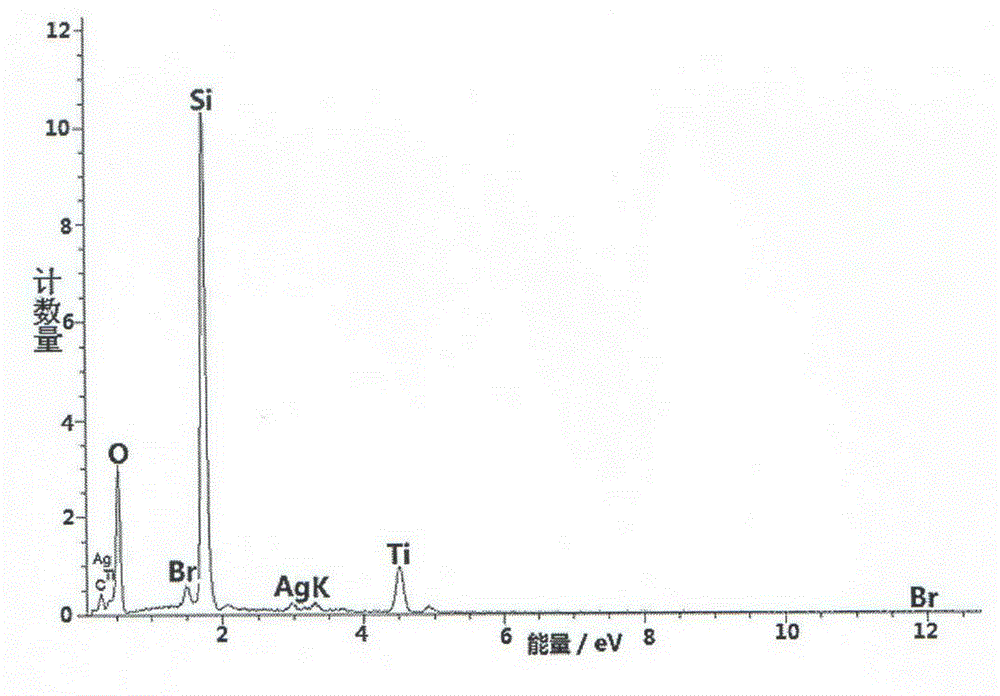
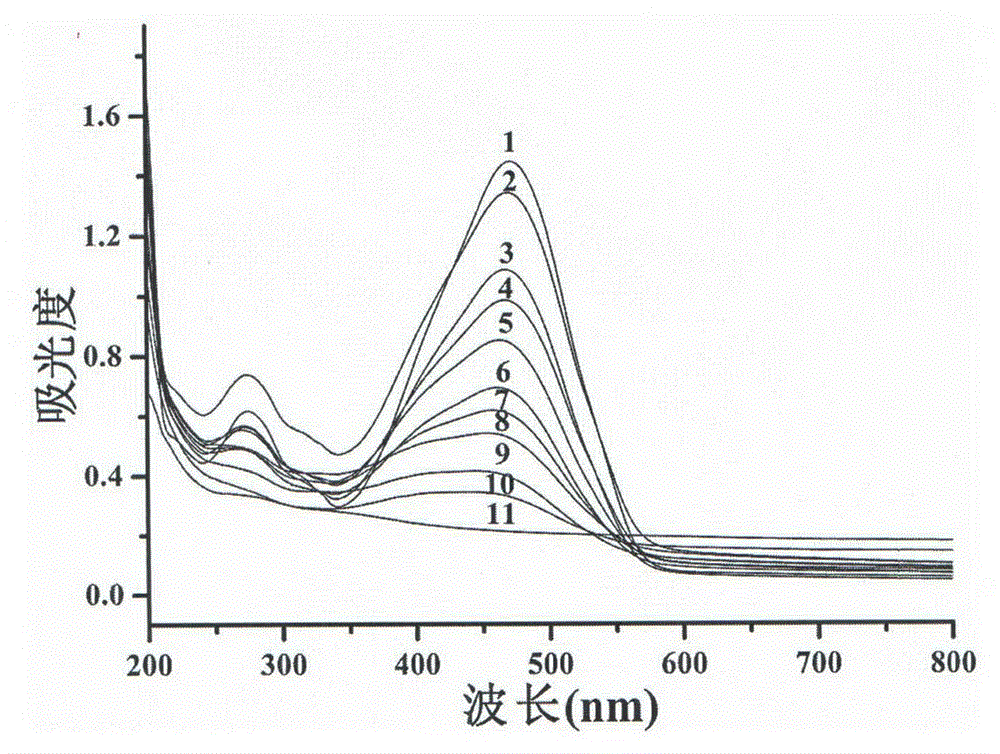
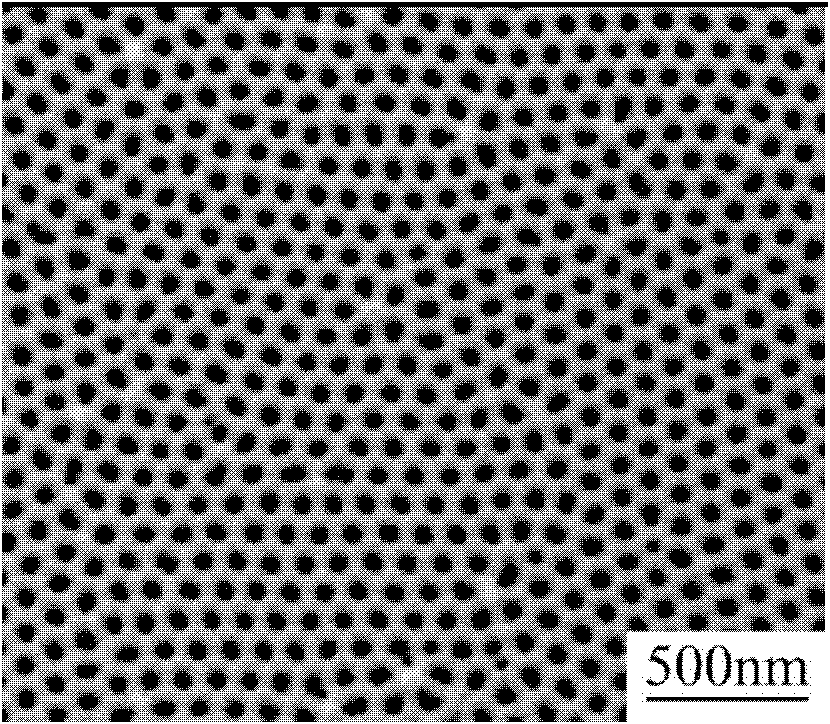
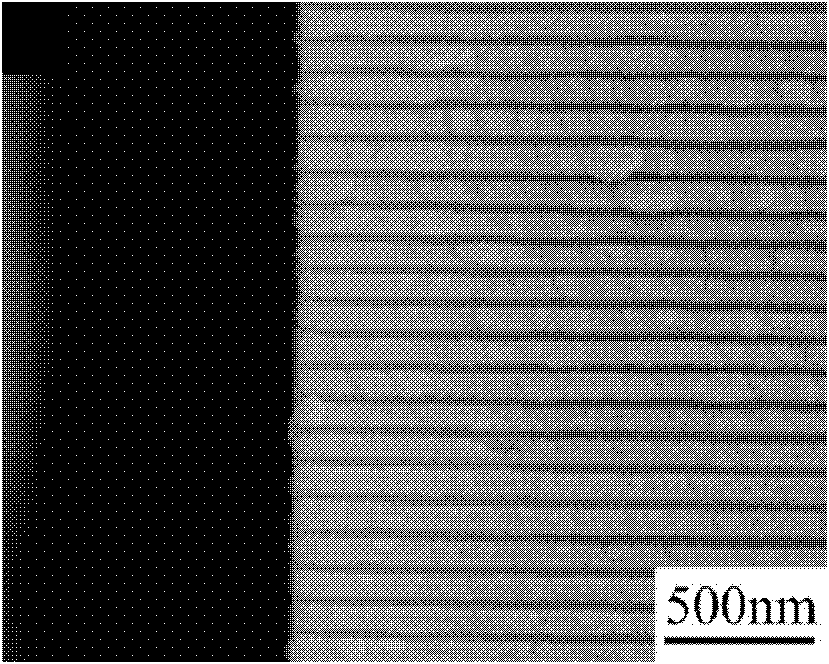
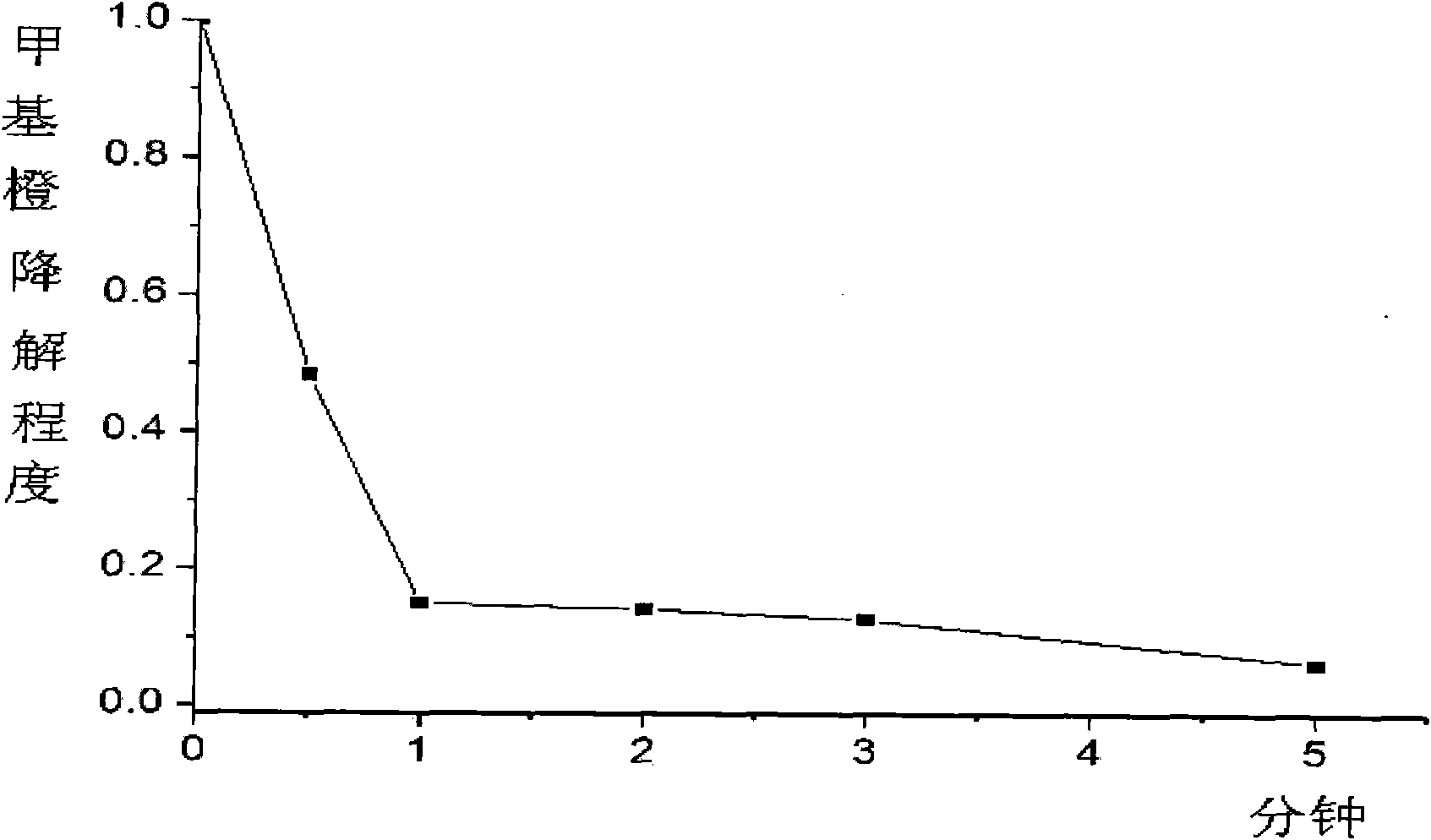
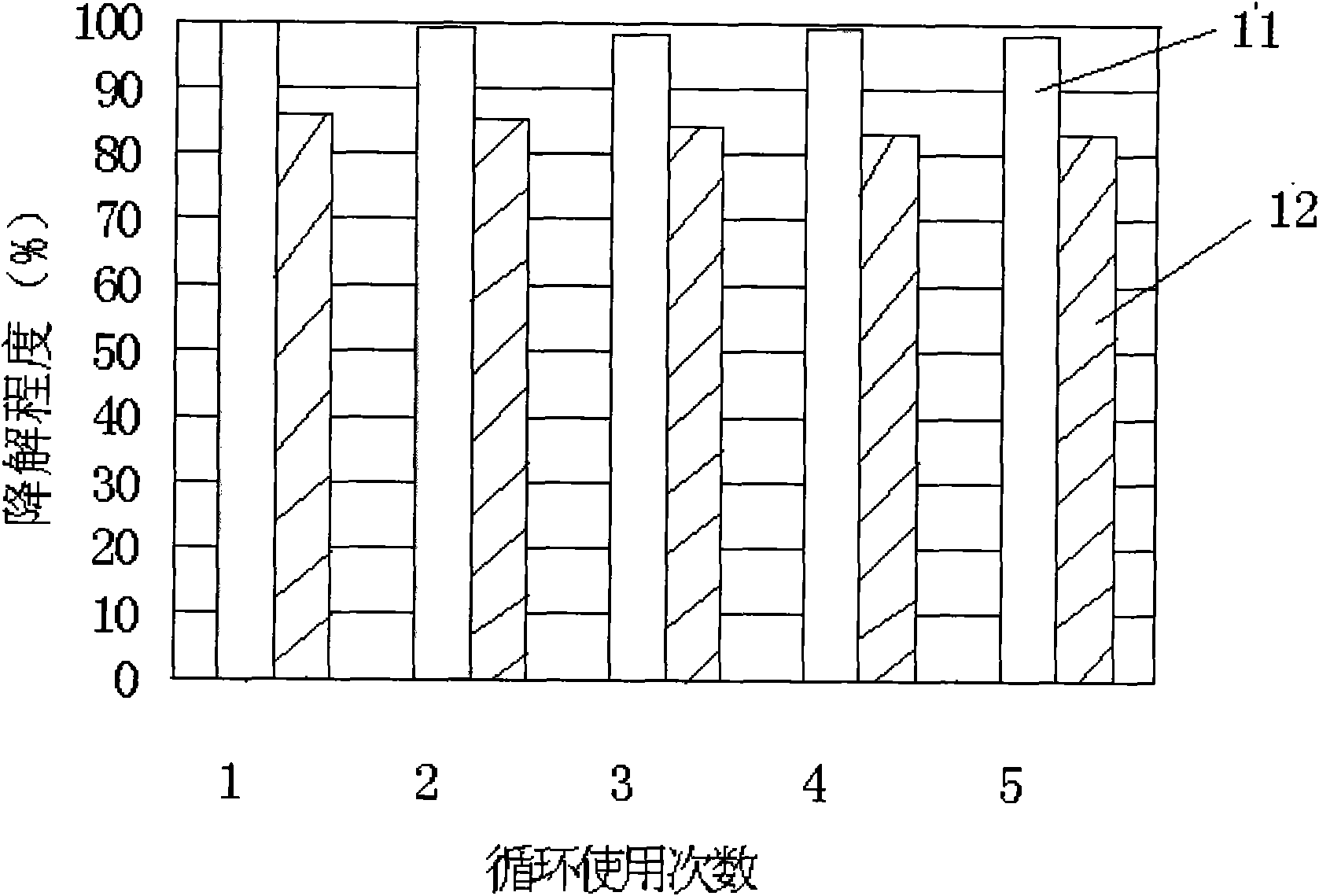
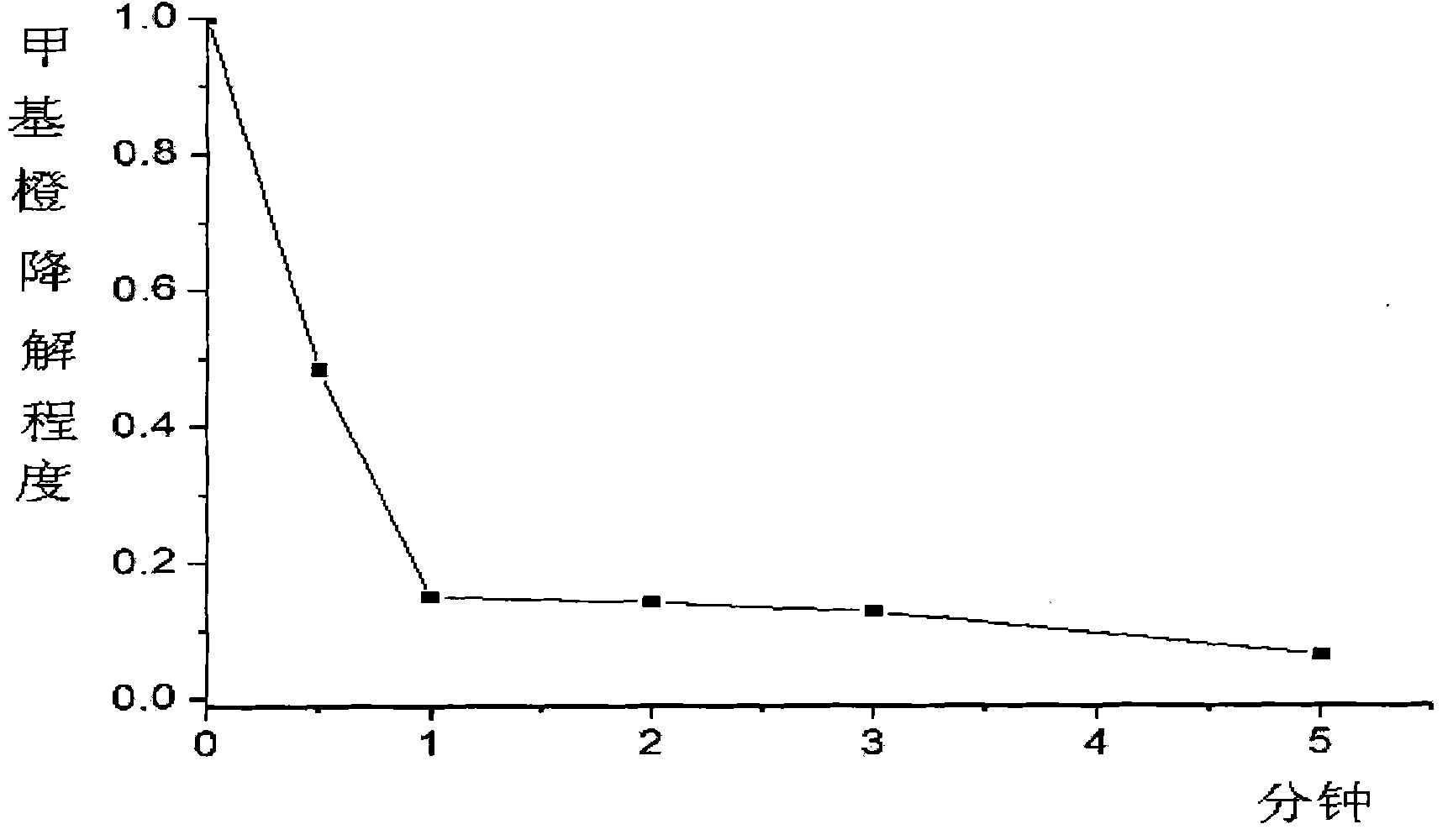
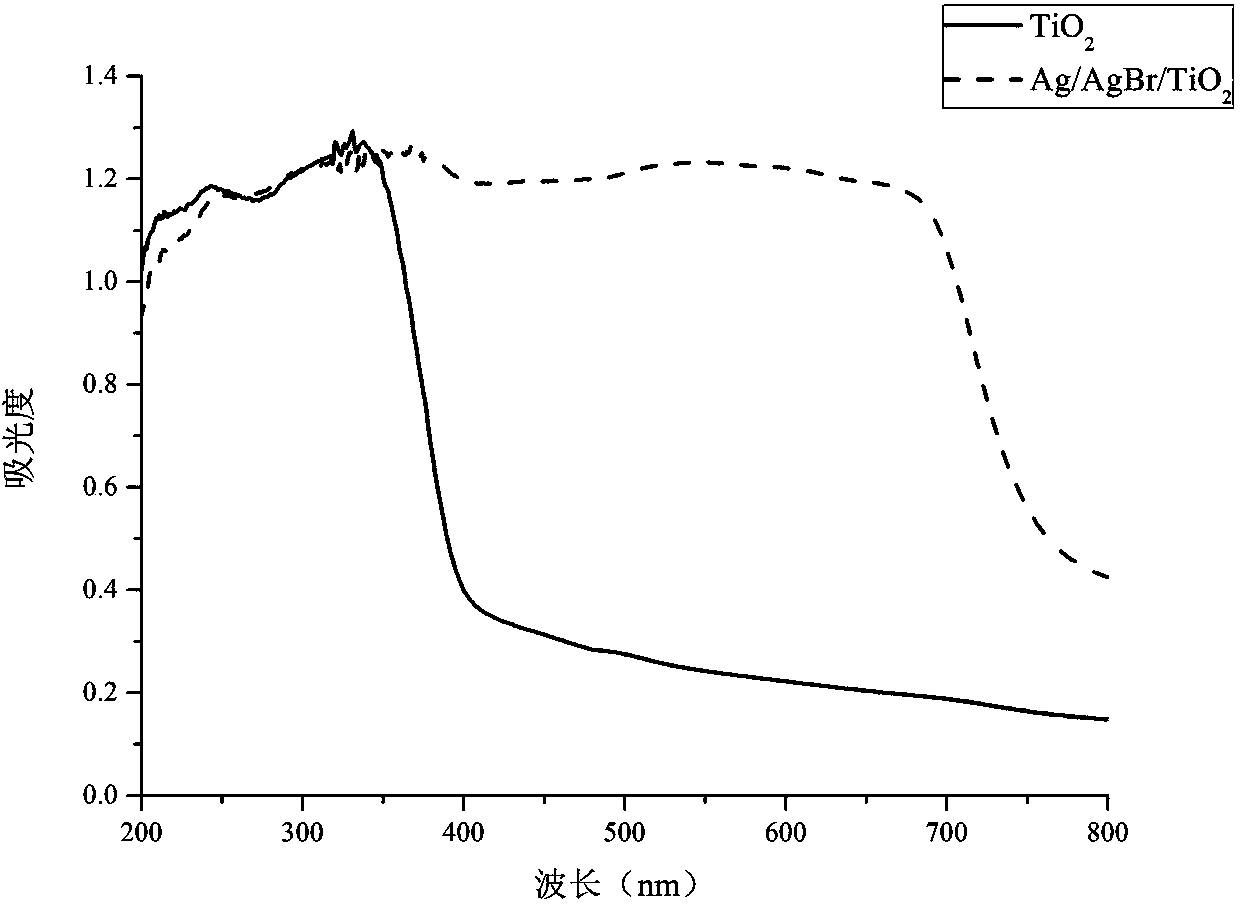
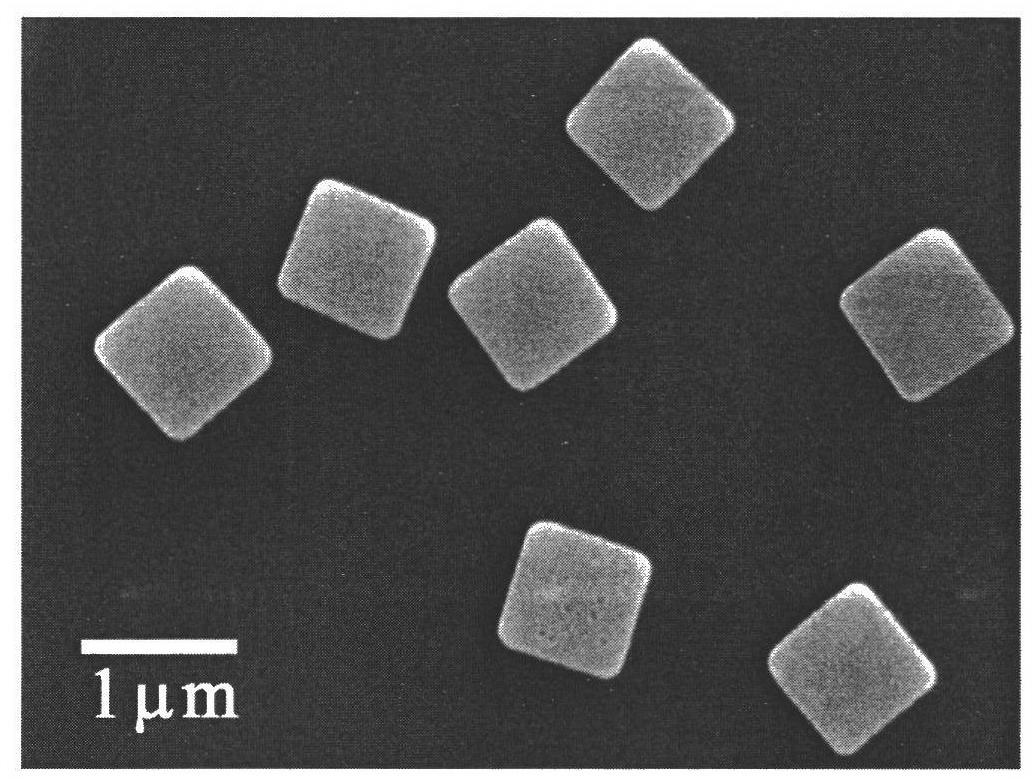
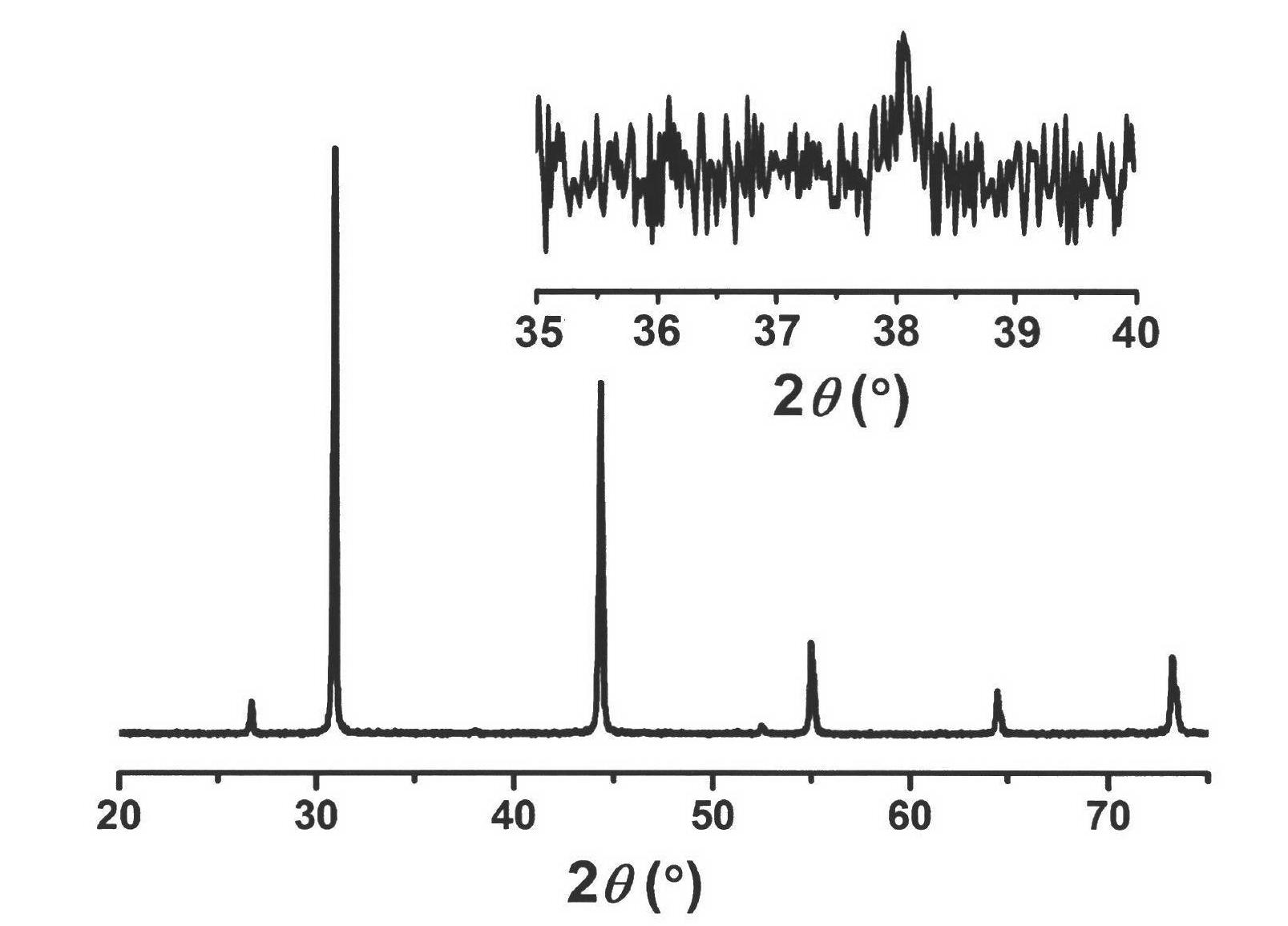
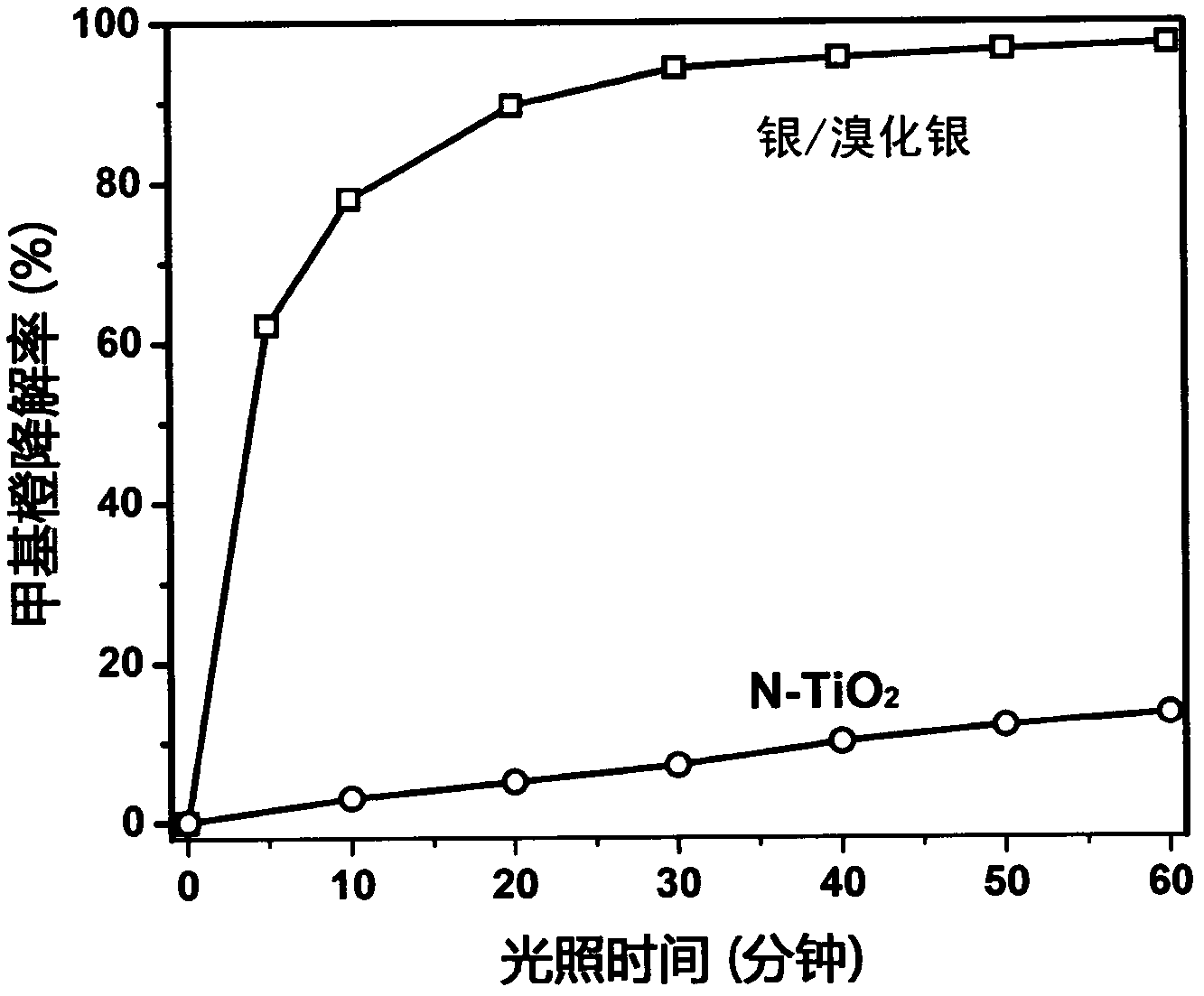
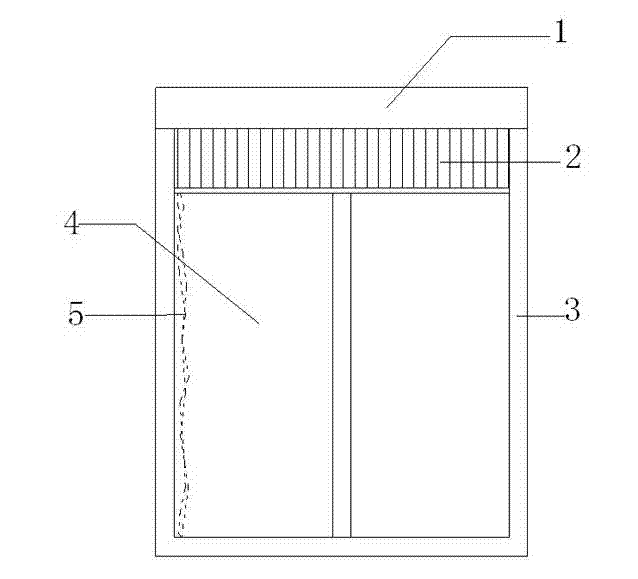
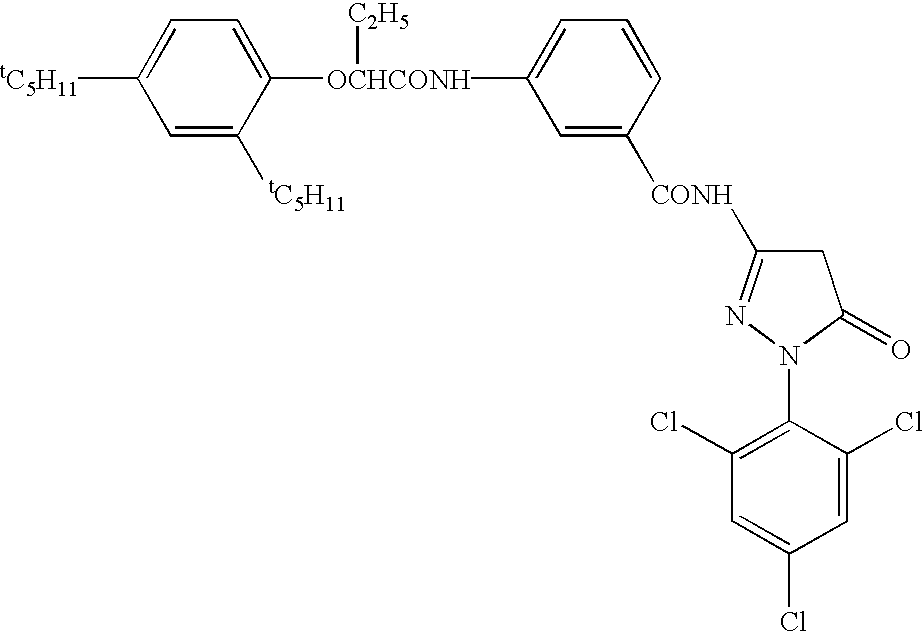
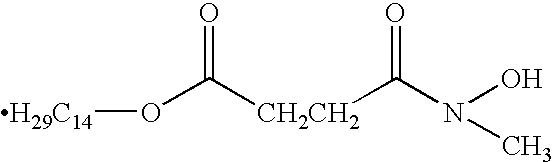
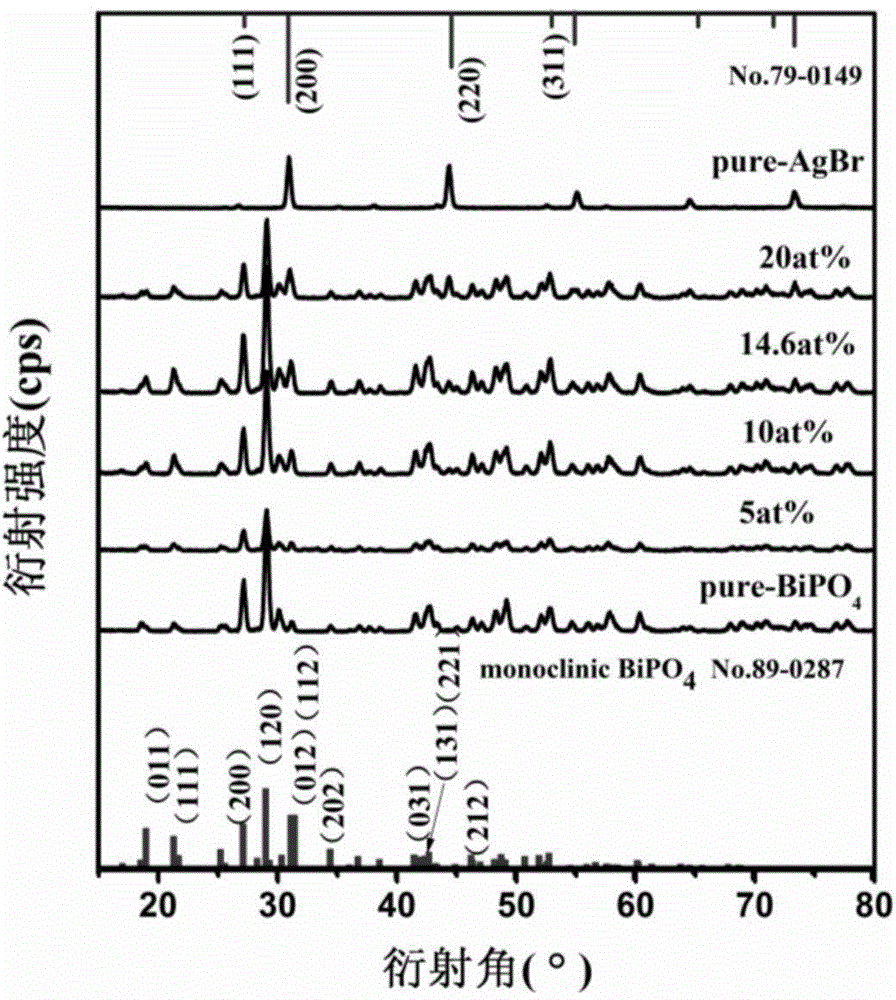
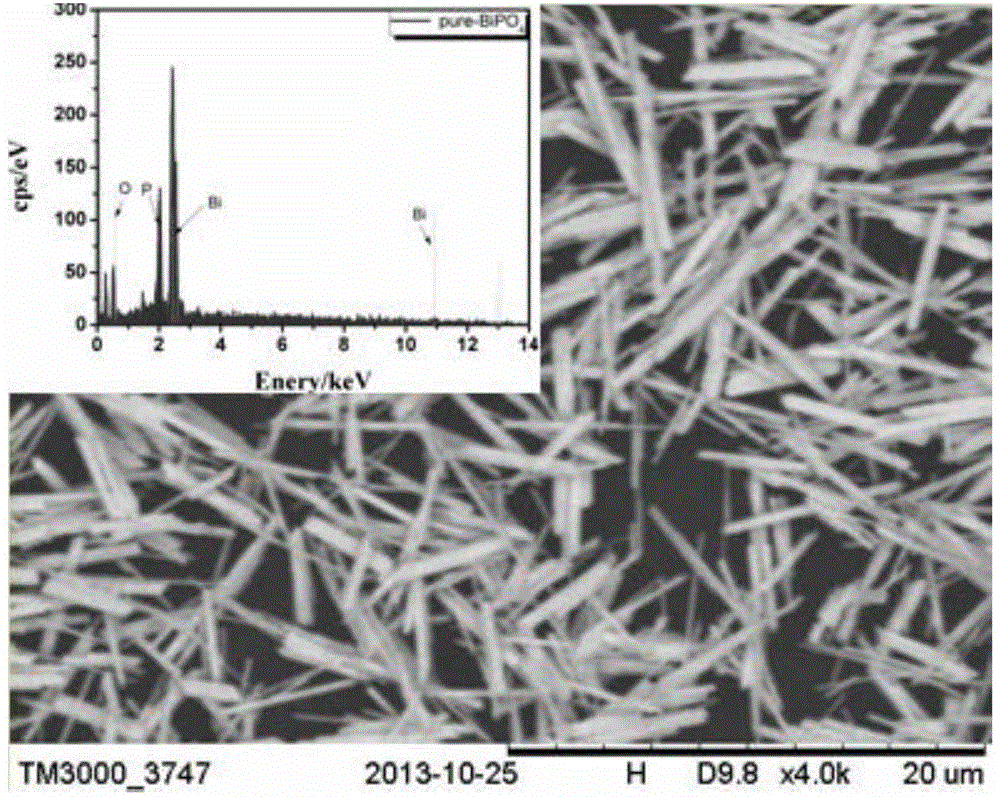
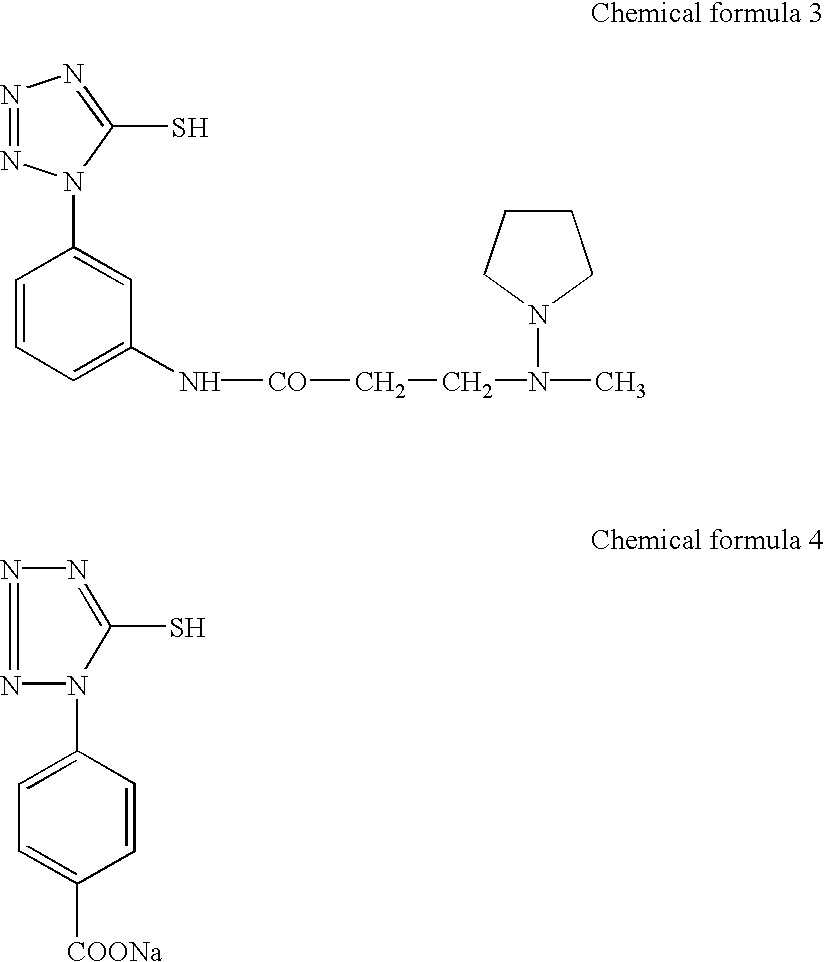
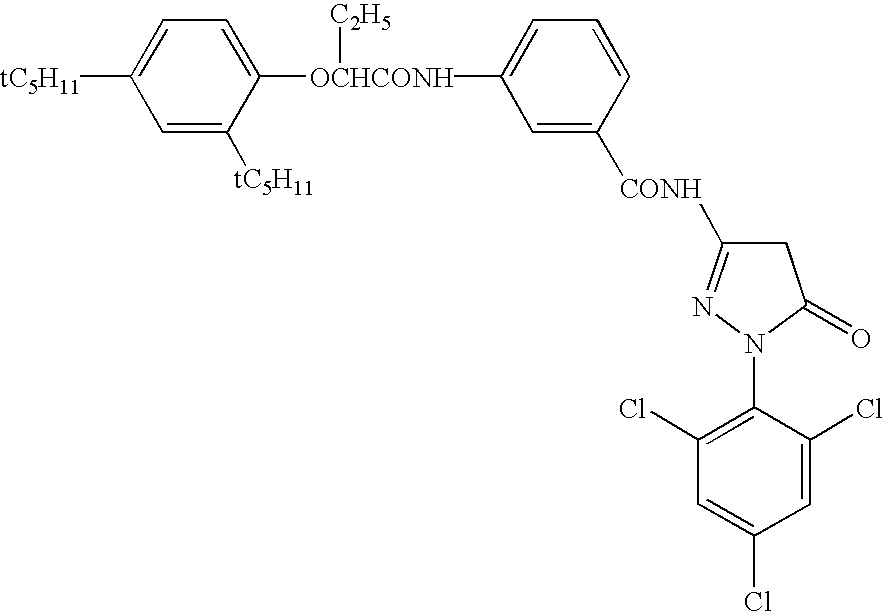
Patents
Literature
242 results about "Silver bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Silver bromide (AgBr), a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite (bromyrite).
Nano silver/silver chloride visible light photocatalysis material and preparation thereof
InactiveCN101279275AAvoid decompositionEfficient use ofWater/sewage treatment by irradiationDispersed particle separationWater bathsSolar light
The present invention provides a nanometer silver / silver chloride visible light optical catalyzing material which consists of nanometer silver and silver chloride with a chemical formula of Ag / AgCl. The nanometer silver particles are loaded on the surface of the silver chloride and occupy 18 to 36 percent of the gross weight. The preparation method includes the following steps: (1) adopting a solid phase sintering method to synthesize silver molybdate, and obtain a mixture of molybdena and silver oxide, tabletting and then sintering; the sintered matter is the silver molybdate; (2) mixing the two according to a proportion of 1g of the silver molybdate and 20ml of concentrated hydrochloric acid and then putting into a high pressure kettle, heating to 150 to 220 DEG C and depositing for 48 to 72 hours; flushing the obtained deposit with a pH value of 7 to obtain the silver bromide; (3) mixing the silver chloride prepared by step (2) with the water solution of silver nitrate, stirring and adding glutamic acid, refluxing under a water bath of 70 DEG C, thus obtaining a nanometer silver / silver chloride optical catalyst. The present invention effectively restrains silver chloride from decomposing by utilizing the plasma effect of the nanometer silver particles and can utilize the energy of solar light more effectively.
Owner:SHANDONG UNIV
Preparation method of glass fiber loaded silver-silver bromide-titanium oxide composite material
ActiveCN103599800ASimple and fast operationEasy to scalePhysical/chemical process catalystsFiberVisible light photocatalytic
The invention provides a preparation method of a glass fiber loaded silver-silver bromide-titanium oxide composite material. The method comprises the following steps: with an organic or inorganic titanium compound as a titanium source and glass fibers as a carrier, obtaining a spherical titanium dioxide (TiO2) nano particle loaded threaded glass fiber composite material by a hydrolysis method under an acidic condition; impregnating the composite material in an ethylene glycol solution containing silver nitrate, and subsequently, adding dropwise an ethylene glycol solution containing potassium bromide to generate an AgBr-TiO2 / glass fiber composite material; and finally, reducing partial Ag<+1> in the AgBr-TiO2 / glass fiber composite material into metal Ag, thus obtaining an Ag-AgBr-TiO2 / glass fiber composite photocatalyst. The method provided by the invention realizes even loading of a nano-material having visible light catalytic activity on the surface of the threaded glass fibers by a two-step method; the method has the advantages of simple and convenient operation, easy large-scale production and the like; the obtained Ag-AgBr-TiO2 / glass fiber composite material has relatively high visible light catalytic activity.
Owner:中科瑞丽分离科技无锡有限公司
Method for preparing silver nanowire array in ordered porous alumina template
InactiveCN102251232AEasy to manufactureLow costSurface reaction electrolytic coatingLiquid/solution decomposition chemical coatingMaterials preparationSilver bromide
The invention belongs to the field of nano material preparation, and discloses a method for preparing a silver nanowire array in an ordered porous alumina template. The method comprises the following steps: (1) sequentially carrying out annealing and ultrasonic cleaning on a high-purity aluminium sheet, removing a natural oxidation film of the high-purity aluminium sheet, sequentially carrying out electrochemical polishing and two-step anodic oxidation on the high-purity aluminium sheet, and removing an unoxidized aluminum substrate and unoxidized through holes of the high-purity aluminium sheet so as to prepare a two-way ordered porous alumina template; (2) filling silver bromide nanowires into the ordered porous alumina template by using a double-chamber chemical deposition method; and (3) irradiating the ordered porous alumina template filled with the silver bromide nanowires by using an ultraviolet lamp so as to carry out photodecomposition on silver bromides, then forming a silver nanowire array in the ordered porous alumina template. By using the method disclosed by the invention, the process for preparing a silver nanowire array is simplified; the method has low requirements on experimental equipment; and the method is simple in operation, low in cost, and favorable for the low-cost fast filling of a silver nanowire array in an ordered porous alumina template.
Owner:TONGJI UNIV
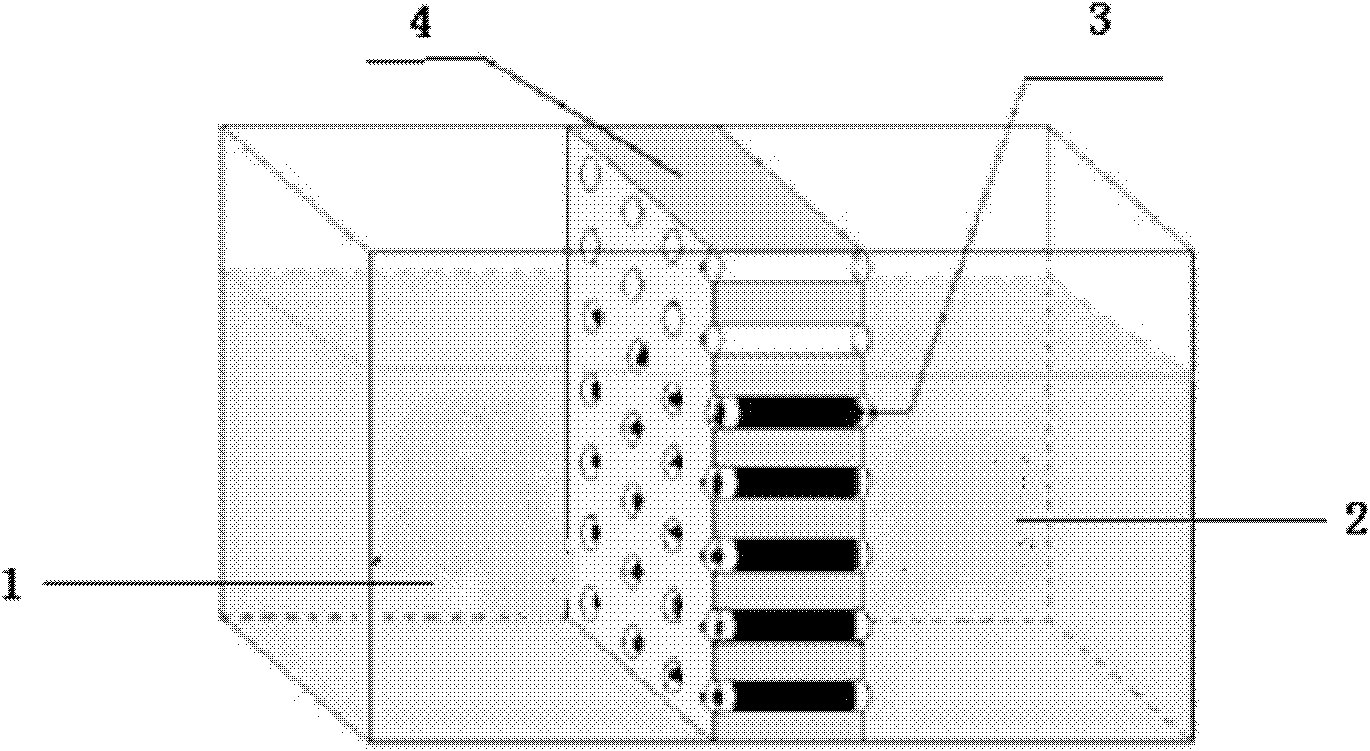
Threshold adjustment for quantum dot array devices with metal source and drain
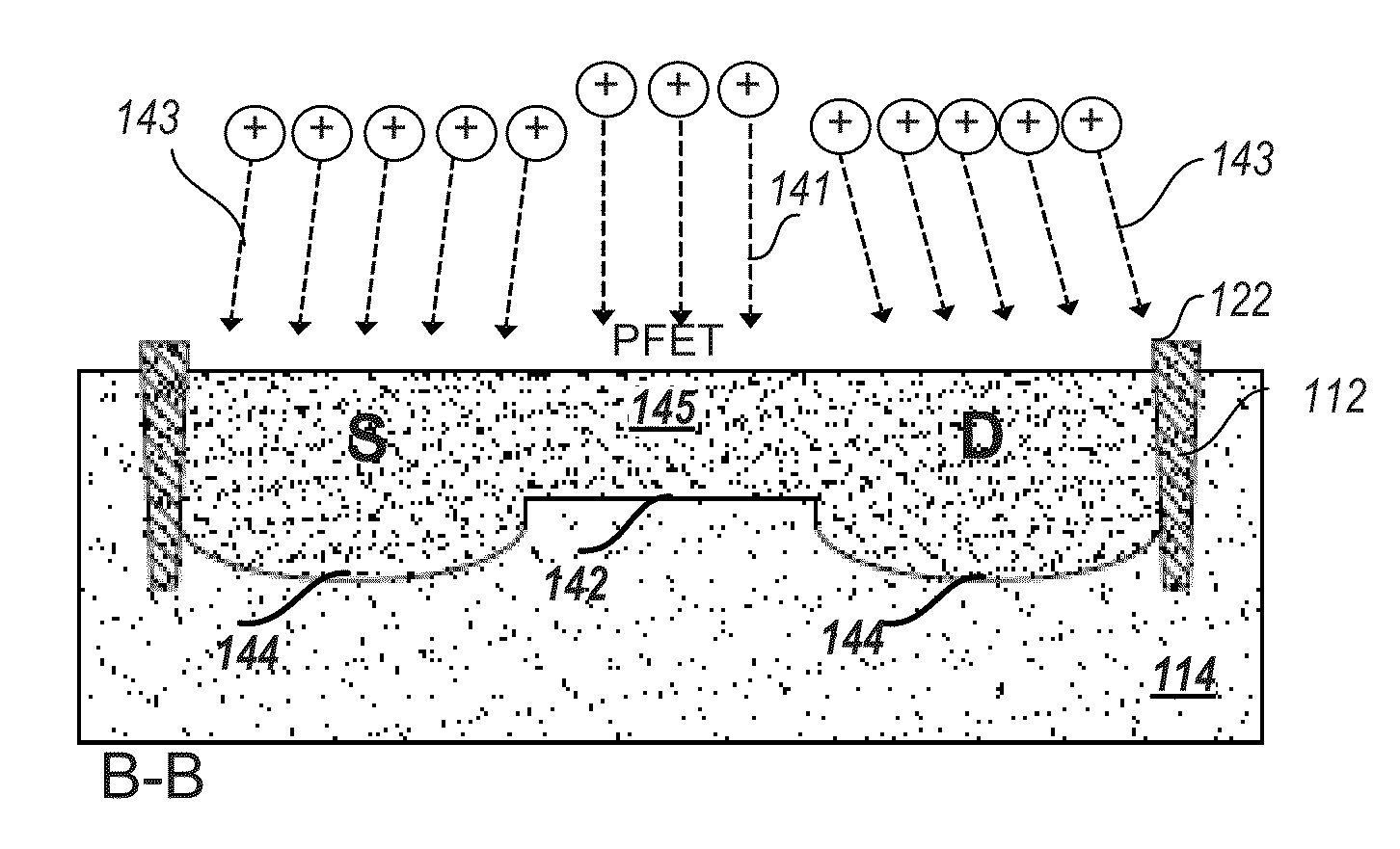
ActiveUS20140084247A1Easy to controlImprove performanceSemiconductor/solid-state device testing/measurementSolid-state devicesMOSFETSilver bromide
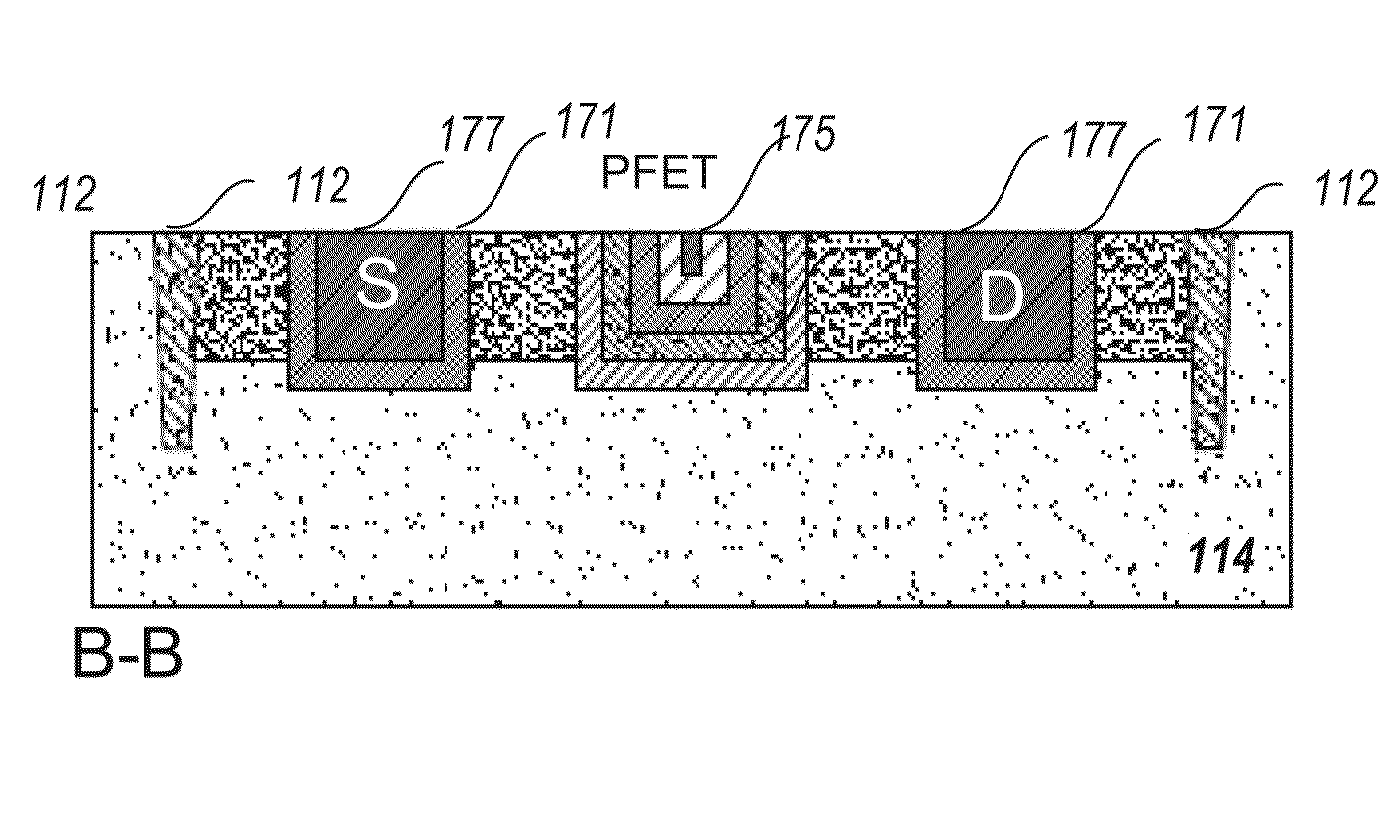
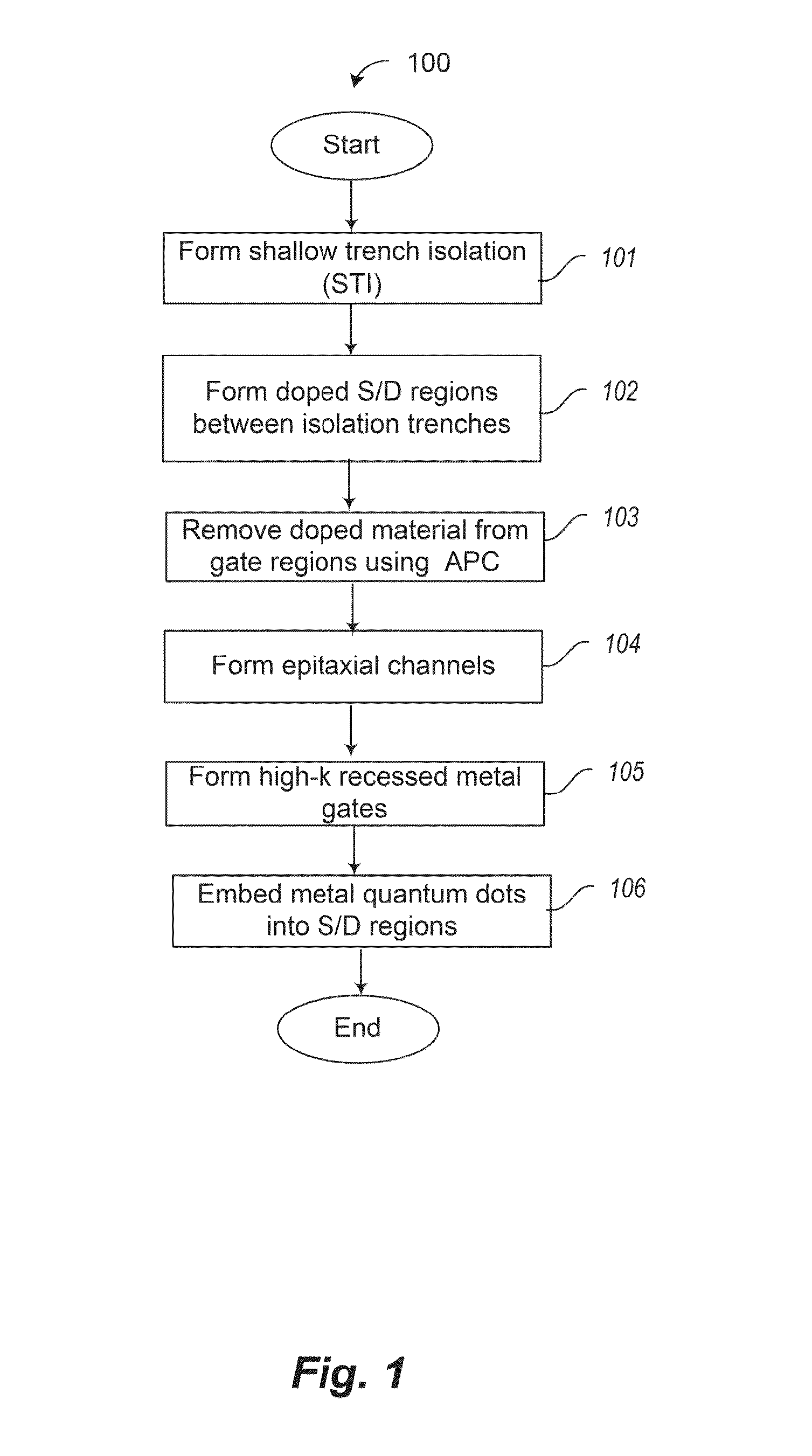
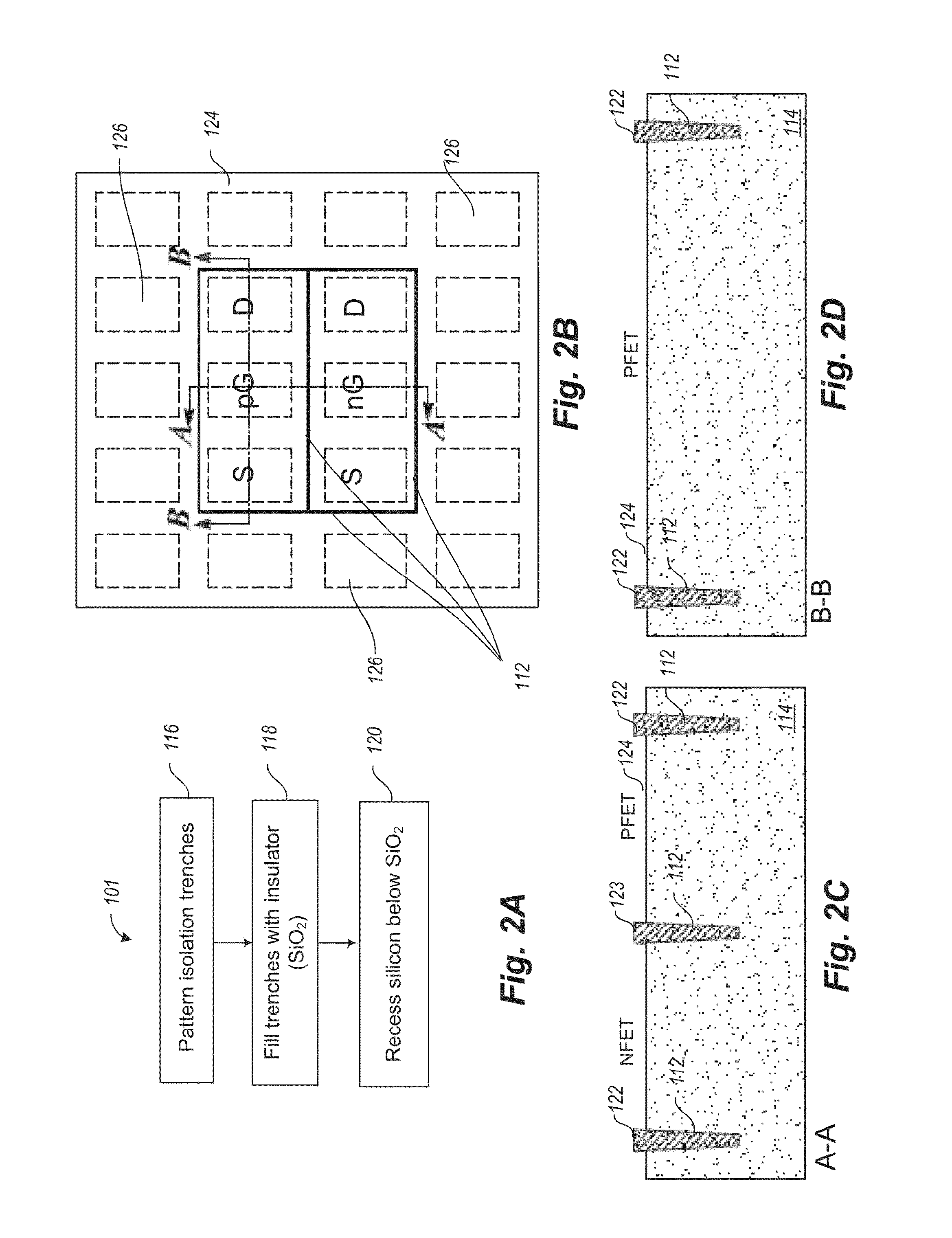
Incorporation of metallic quantum dots (e.g., silver bromide (AgBr) films) into the source and drain regions of a MOSFET can assist in controlling the transistor performance by tuning the threshold voltage. If the silver bromide film is rich in bromine atoms, anion quantum dots are deposited, and the AgBr energy gap is altered so as to increase Vt. If the silver bromide film is rich in silver atoms, cation quantum dots are deposited, and the AgBr energy gap is altered so as to decrease Vt. Atomic layer deposition (ALD) of neutral quantum dots of different sizes also varies Vt. Use of a mass spectrometer during film deposition can assist in varying the composition of the quantum dot film. The metallic quantum dots can be incorporated into ion-doped source and drain regions. Alternatively, the metallic quantum dots can be incorporated into epitaxially doped source and drain regions.
Owner:STMICROELECTRONICS SRL
High-efficiency nano silver/silver bromide sunshine photocatalytic material and preparation method thereof
InactiveCN101816943AStrong absorption capacitySimple preparation processPhysical/chemical process catalystsWater/sewage treatment by irradiationSilver bromideMicron size
The invention discloses a high-efficiency nano silver / silver bromide sunshine photocatalytic material and a preparation method thereof. The material consists of the nano silver and the micron-sized silver bromide, and the chemical formula is Ag / AgBr; and the nano silver is uniformly distributed on the surface of the silver bromide, the grain size of the silver bromide is 1 to 3 mu m, and the grain size of the silver bromide is 8 to 14 nm. Compared with the prior art, the prepared nano silver / silver bromide sunshine photocatalytic material can be used for purifying pollutants in water and sterilizing; the effect is much better than that of the conventional commonly used photocatalytic material P25 and a recently developed 'Wang catalyst'; the material can directly use solar energy with high efficiency, the photocatalytic ability is almost not decreased after being recycled for many times; and meanwhile, the nano silver / silver bromide sunshine photocatalytic material has the advantages of simple and convenient preparation process, low cost, and suitability for industrial production.
Owner:ANHUI NORMAL UNIV
Nano silver/silver bromide visible light photocatalysis material and preparation thereof
InactiveCN101279274AAvoid decompositionImprove conductivityWater/sewage treatment by irradiationDispersed particle separationWater bathsSolar light
The present invention provides a nanometer silver / silver bromide visible light optical catalyzing material which consists of nanometer silver and silver bromide with a chemical formula of Ag / AgBr. The nanometer silver particles are loaded on the surface of the silver bromide and occupy 23.5 to 47 percent of the gross weight. The preparation method includes the following steps: (1) adopting a solid phase sintering method to synthesize silver molybdate, and obtain a mixture of molybdena and silver oxide, tabletting and then sintering; the sintered matter is the silver molybdate; (2) mixing the two according to a proportion of 1g of the silver molybdate and 20ml of acid hydrobromic and then putting into a high pressure kettle, heating to 150 to 220 DEG C and depositing for 48 to 72 hours; flushing the obtained deposit with a pH value of 7 to obtain the silver bromide; (3) mixing the silver bromide with the water solution of silver nitrate, adding glutamic acid after stirring, refluxing under a water bath of 70 DEG C, thus obtaining a nanometer silver / silver bromide optical catalyst. The present invention effectively restrains silver chloride from decomposing by utilizing the plasma effect of the nanometer silver particles and can utilize the energy of solar light more effectively.
Owner:SHANDONG UNIV
Production method of durable combined antimicrobial non-woven fabric material
The invention provides a method for making a durable compound antibiosis nonwoven cloth material. By performing an efficient combination of insoluble silver particle pincer system which has a non dissoluble type high efficient antibiosis function with dissoluble type chlorine system quaternary ammonium salt, an antimicrobial solution with a certain concentration is made, by performing immersing and rolling finishing on the chemical fiber non woven cloth with the antimicrobial, a non woven cloth which combines active antibiosis and passive antibiosis functions is made, the non dissoluble silver pincer complex is a nanometer particle pincer complex of silver carbonate or silver bromide; the quaternary ammonium salt is a CTMAC or a double stearyl DHTDMAC, which has a good surface treating and antibiosis performance; as for the mixture ratio of the two, the former is 10 to 60 percent, the latter is 90 to 40 percent, and as for the optimal mixture ratio, the former is 25 percent and the latter is 75 percent. The after finishing is adding the assembled antimicrobial onto the molded non woven cloth coiled material by immersing and sprinkling, and baking and sizing the material, without any other surface pre-treating process.
Owner:福建鑫华股份有限公司
Preparation method of 3D-shaped silver/silver bromide/titanium dioxide catalyst
The invention relates to a preparation method of a 3D-shaped silver / silver bromide / titanium dioxide catalyst. The preparation method comprises the following steps: firstly mixing titanate with concentrated hydrochloric acid, and dropwise adding a water solution of cetyl trimethyl ammonium bromide into a titanate solution; respectively adding ethanol and urea into a mixed solution of the titanate and cetyl trimethyl ammonium bromide, uniformly mixing, and carrying out hydrothermal reaction at 150 DEG C; adding silver nitrate and ammonium hydroxide into turbid liquid obtained in the hydrothermal reaction, stirring for 24 hours, carrying out centrifugal washing and drying, and roasting for 2 hours at 400 DEG C; carrying out reduction on roasted samples for 3 hours under ultraviolet light, so as to obtain the 3D-shaped silver / silver bromide / titanium dioxide catalyst. The preparation method has the beneficial effects that the process is simple, requirements on equipment are low, and the prepared titanium dioxide photocatalyst has strong response under a visible light condition.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Nano silver/cubic silver bromide photocatalysis material and preparation method thereof
InactiveCN102416335ASimple processShort preparation cycleMaterial nanotechnologyPhysical/chemical process catalystsWarm waterControllability
The invention relates to a nano silver / cubic silver bromide photocatalysis material and a preparation method thereof. The photocatalysis material is formed by homogeneous cubic silver bromide microcrystals and nano silver formed on the surfaces of the microcrystals in situ, wherein the nano silver particles account for 3-25% of the total catalyst by weight. The preparation method comprises the following steps of: (1) in the presence of a polymer protectant, adopting a double jet-precipitation method and controlling the pBr value of the reaction process to prepare the cubic silver bromide microcrystals; (2) removing the polymer protectant and inorganic salts by washing the cubic silver bromide microcrystals with warm water; and (3) using an ultraviolet lamp to carry out photoreduction treatment on the prepared silver bromide microcrystals, thus obtaining the nano silver / cubic silver bromide photocatalysis material. Compared with the prior art, the preparation method has the advantages of simple preparation process, high temperature dispensability and catalyst morphology controllability. The prepared catalyst can absorb visible light through the silver nanoparticle plasmon resonance effect and efficiently degrade such organic pollutants as dyes.
Owner:EAST CHINA UNIV OF SCI & TECH
Intelligent photochromic window and use method thereof
InactiveCN103032018AChange light transmittanceImprove the lighting environmentLight protection screensSilver bromideShutter
The invention relates to an intelligent photochromic window and a use method thereof, belonging to the technical field of building of architecture. The intelligent photochromic window comprises a window frame and photochromic glass, wherein the photochromic glass is arranged in the window frame; the outer top of the window frame is provided with a roller shutter shaft; a shading roller shutter is arranged on the roller shutter shaft; the photochromic glass indicates composite glass formed by compositely smearing silver bromide and copper oxide on normal glass; the shading roller shutter is a normal shade cloth roller shutter; and one end of the roller shutter shaft is provided with a pull rope. According to the intelligent photochromic window, the light transmittance of the window can be changed along with intensity of sunlight, strong sunlight in summer is avoided, the light environment in the window is improved, and the comfort of a human body is enhanced; and the intelligent photochromic window is easy and convenient to produce and mount.
Owner:SHAOXING UNIVERSITY
Atomic layer deposition of selected molecular clusters
ActiveUS20150053930A1Different energy band gapIncrease productionVacuum evaporation coatingSemiconductor/solid-state device manufacturingSemiconductor materialsMolecular cluster
Energy bands of a thin film containing molecular clusters are tuned by controlling the size and the charge of the clusters during thin film deposition. Using atomic layer deposition, an ionic cluster film is formed in the gate region of a nanometer-scale transistor to adjust the threshold voltage, and a neutral cluster film is formed in the source and drain regions to adjust contact resistance. A work function semiconductor material such as a silver bromide or a lanthanum oxide is deposited so as to include clusters of different sizes such as dimers, trimers, and tetramers, formed from isolated monomers. A type of Atomic Layer Deposition system is used to deposit on semiconductor wafers molecular clusters to form thin film junctions having selected energy gaps. A beam of ions contains different ionic clusters which are then selected for deposition by passing the beam through a filter in which different apertures select clusters based on size and orientation.
Owner:STMICROELECTRONICS SRL
Threshold adjustment for quantum dot array devices with metal source and drain
ActiveUS20160111521A1Easy to controlImprove performanceSemiconductor/solid-state device testing/measurementSolid-state devicesMOSFETAtomic layer deposition
Incorporation of metallic quantum dots (e.g., silver bromide (AgBr) films) into the source and drain regions of a MOSFET can assist in controlling the transistor performance by tuning the threshold voltage. If the silver bromide film is rich in bromine atoms, anion quantum dots are deposited, and the AgBr energy gap is altered so as to increase Vt. If the silver bromide film is rich in silver atoms, cation quantum dots are deposited, and the AgBr energy gap is altered so as to decrease Vt. Atomic layer deposition (ALD) of neutral quantum dots of different sizes also varies Vt. Use of a mass spectrometer during film deposition can assist in varying the composition of the quantum dot film. The metallic quantum dots can be incorporated into ion-doped source and drain regions. Alternatively, the metallic quantum dots can be incorporated into epitaxially doped source and drain regions.
Owner:STMICROELECTRONICS SRL
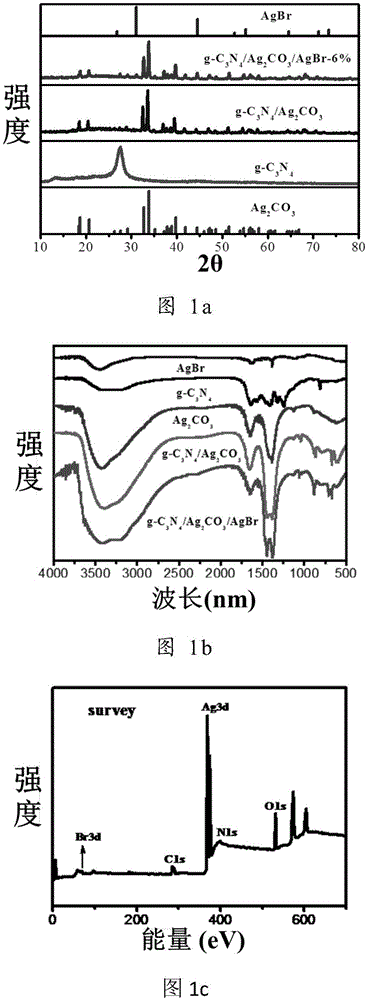
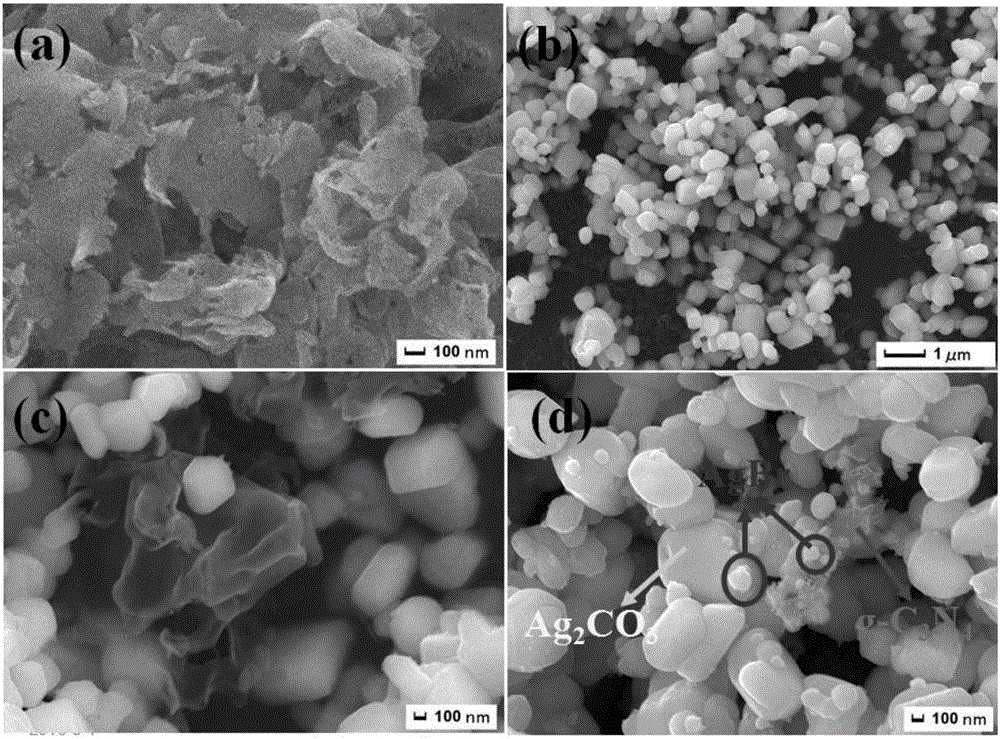
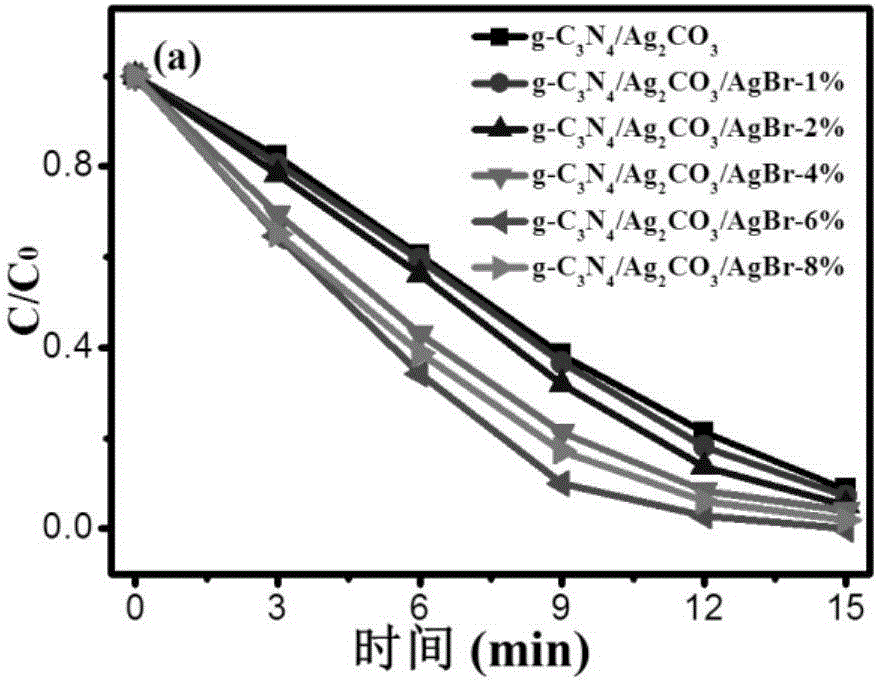
A graphitic carbon nitride/silver carbonate/silver bromide ternary composite nano-material, a preparing method thereof and uses of the nano-material
InactiveCN106378170AHigh yieldImprove photocatalytic performancePhysical/chemical process catalystsSilver carbonateCarbonate
A graphitic carbon nitride / silver carbonate / silver bromide ternary composite nano-material, a preparing method thereof and uses of the nano-material are disclosed. Rodlike Ag<2>CO3 and g-C3N4 having a lamellar structure grow together, and AgBr particles are attached to the surface of the Ag<2>CO3. The nano-material comprises 5-30 wt% of the g-C3N4 and 1-8 wt% of the AgBr, with the balance being the Ag<2>CO3. The preparing method includes (1) subjecting urea and melamine to high-temperature solid-phase sintering to obtain a g-C3N4 lamellar structure, (2) synthesizing the g-C3N4 nanosheet, a soluble silver salt and a carbonate or bicarbonate into g-C3N4 / Ag<2>CO3 particles through a solution precipitation reaction, and (3) introducing Br<-> to the g-C3N4 / Ag<2>CO3 particles. The prepared ternary composite nano-material is high in yield and can be adopted as a photocatalyst.
Owner:ZHENJIANG COLLEGE
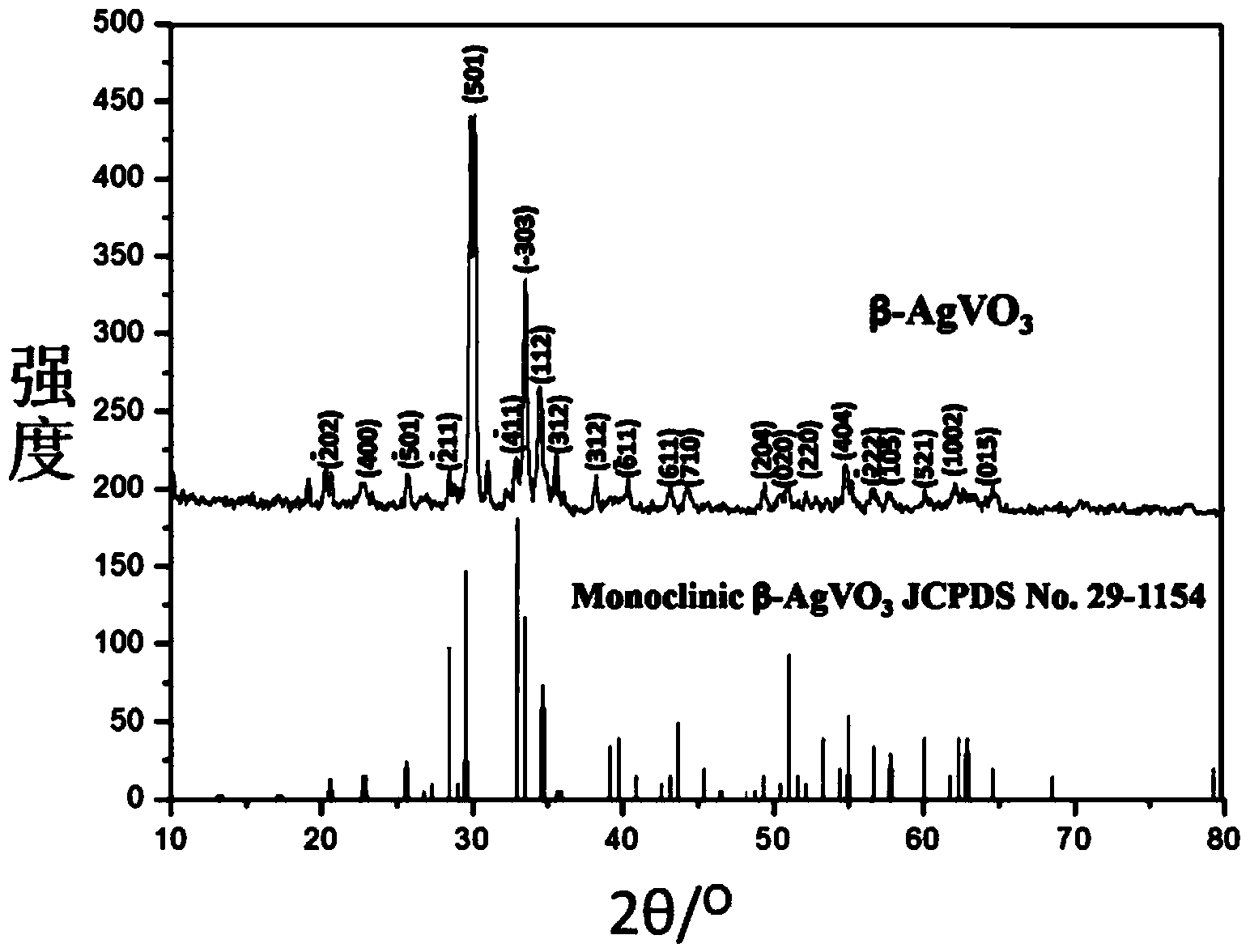
Silver/silver bromide/silver metavanadate plasma compound photocatalyst and preparation method thereof
InactiveCN103846096AGood photodegradation effectHigh yieldPhysical/chemical process catalystsSilver bromideAmmonium bromide
The invention discloses a silver / silver bromide / silver metavanadate plasma compound photocatalyst and a preparation method thereof. The photocatalyst comprises silver, silver bromide and silver metavanadate, wherein the silver metavanadate is nanoribbon, and silver and silver bromide particles are loaded on the silver metavanadate. The preparation method comprises steps of fully dissolving silver metavanadate and dodecyl trimethyl ammonium bromide in water; continuously stirring under light condition and fully mixing; and finally irradiating in the sun so as to obtain the silver / silver bromide / silver metavanadate plasma compound photocatalyst. The preparation method has the advantages of high yield, low cost and short production process, and can be put into industrial production conveniently. The silver / silver bromide / silver metavanadate plasma compound photocatalyst prepared by the method has very excellent light degradation performance.
Owner:ANHUI NORMAL UNIV
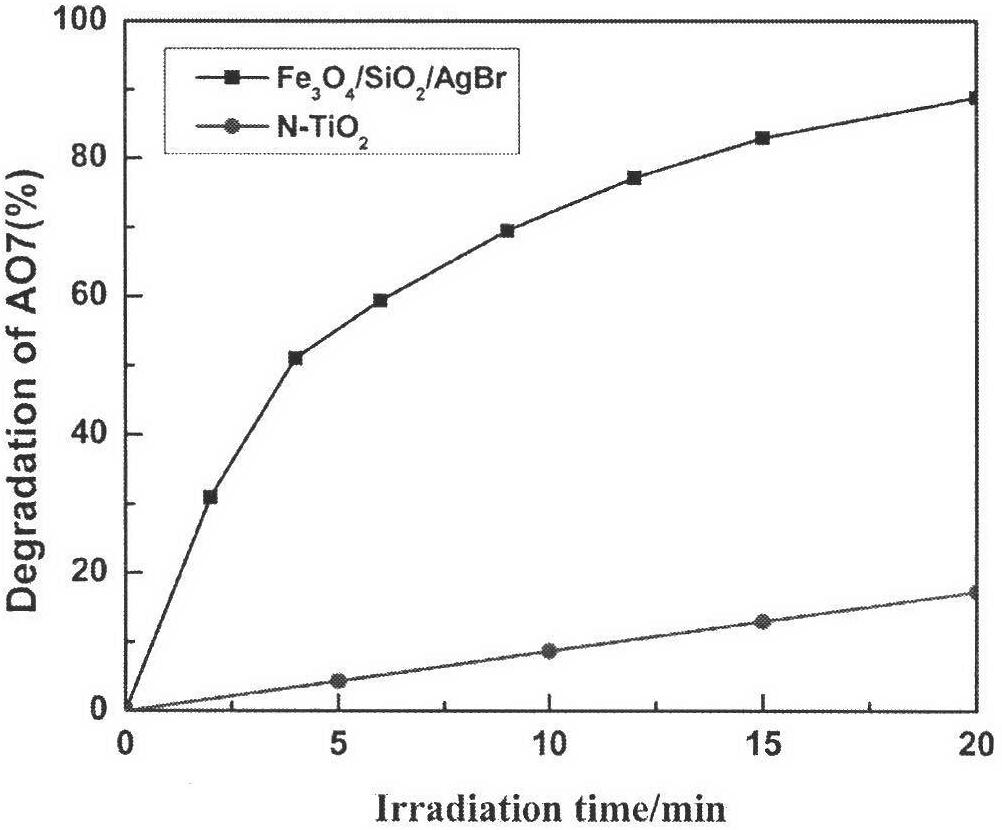
Magnetically supported silver bromide photochemical catalysis material and preparation method thereof
InactiveCN102671674AMetal/metal-oxides/metal-hydroxide catalystsPolyethylene glycolUltraviolet lights
The invention relates to a magnetically supported silver bromide photochemical catalysis material and a preparation method thereof. The photochemical catalysis material is composed of a ferroferric oxide / silicon dioxide nuclear shell structure and a silver bromide supported by an outer layer of the ferroferric oxide / silicon dioxide nuclear shell structure. The preparation method comprises the steps: (1) using perchloride iron as a precursor, using polyethylene glycol as a dispersing agent, using a ethylene glycol as a high-boiling-point solvent and utilizing a hydrothermal method to prepare monodisperse magnetic ferroferric oxide nanometer particles; (2) cladding silicon dioxide to form the ferroferric oxide / silicon dioxide nuclear shell structure; and (3) using silver nitrate as a silver source, using potassium bromide or other bromides as a bromine source, supporting silver bromide on the ferroferric oxide / silicon dioxide surface, and using ultraviolet light to perform photo-reduction to silver bromide microcrystal to enable the surface to form silver nanometer particles in a in-situ mode. Compared with existing methods, the magnetically supported silver bromide photochemical catalysis material and the preparation method have the advantages that a catalyst is controllable in morphology and can be recycled in a magnetic control mode and the like. The catalyst prepared by means of the method can efficiently degrade organic pollutants including dyestuffs and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
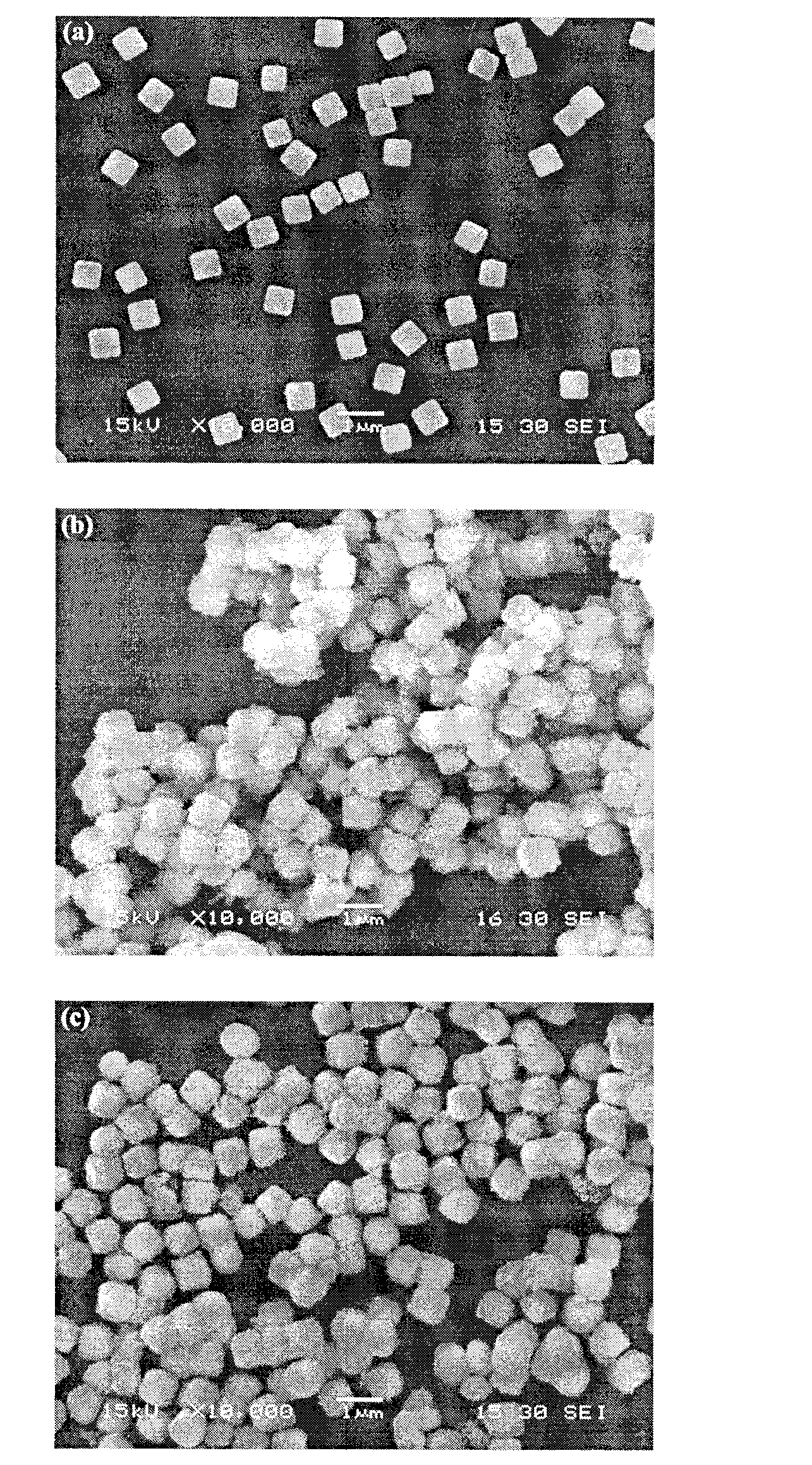
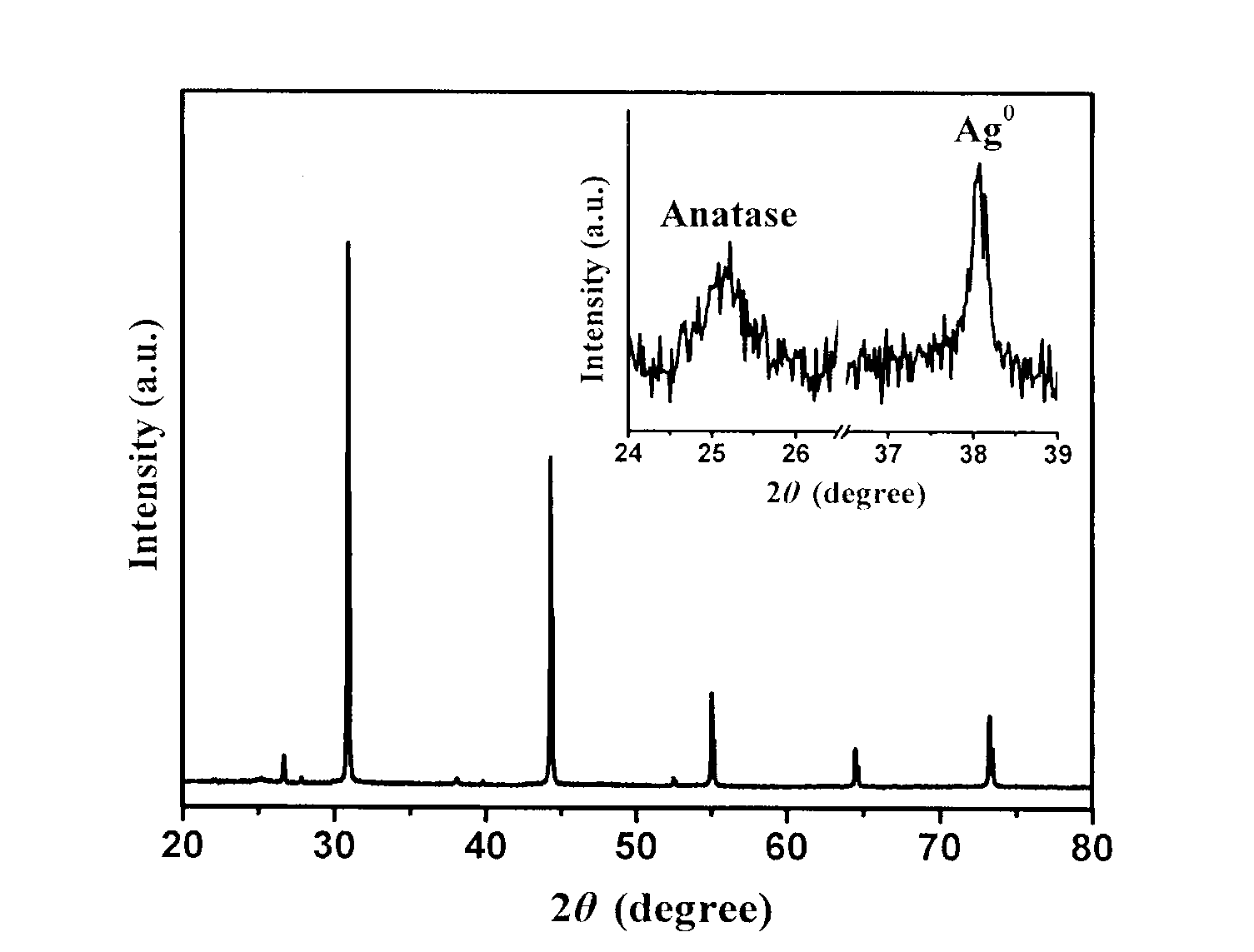
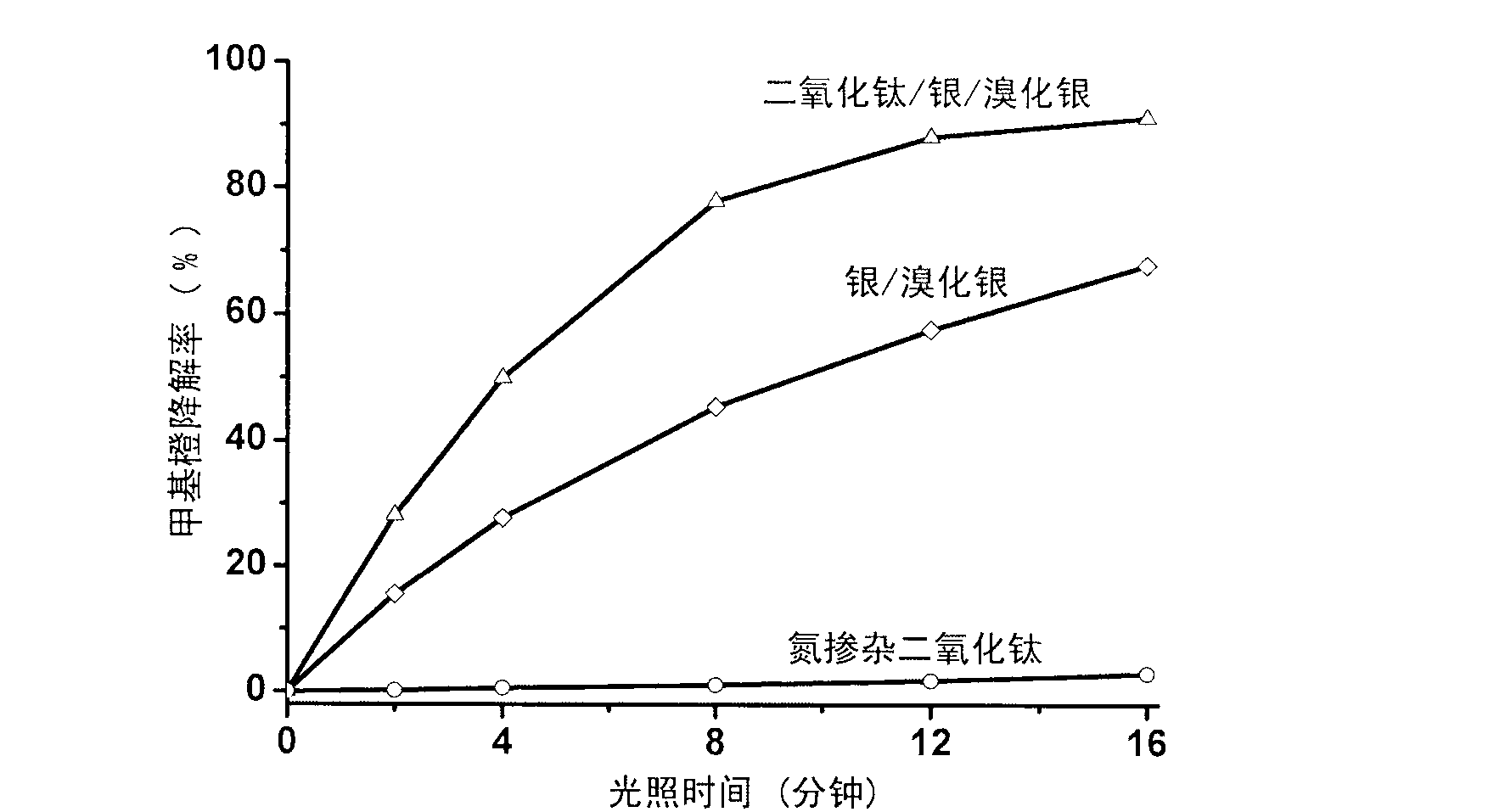
Titanium dioxide/silver/silver bromide core-shell photocatalyst and preparation method thereof
InactiveCN102909039AEfficient degradationSimple processPhysical/chemical process catalystsWater/sewage treatment by irradiationSilver bromideUltraviolet
The invention relates to titanium dioxide / silver / silver bromide core-shell photocatalyst and a preparation method thereof. A core of the catalyst consists of homogeneous cubic silver bromide and nano-sliver formed in situ on the surface of the homogeneous cubic silver bromide, and a shell of the catalyst consists of titanium dioxide coating. The preparation method includes the steps of firstly, preparing cubic silver bromide microcrystalline by double-column precipitation in the presence of polymeric protector; coating titanium dioxide to the surface of silver bromide by hydrothermal process in the presence of polymeric protector; thirdly, subjecting the prepared titanium dioxide / silver bromide to photo-reduction by ultraviolet lamps to obtain the titanium dioxide / silver / silver bromide core-shell photocatalyst. Compared with the prior art, the titanium dioxide / silver / silver bromide core-shell photocatalyst has the advantages that the preparation process is simple, the catalyst is morphologically controllable, and agglomeration and photo-corrosion of the silver bromide are avoided effectively. The prepared catalyst absorbs visible light to efficiently degrade organic pollutants such as dye by resonance effect of ions of silver bromide, nano-sliver and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
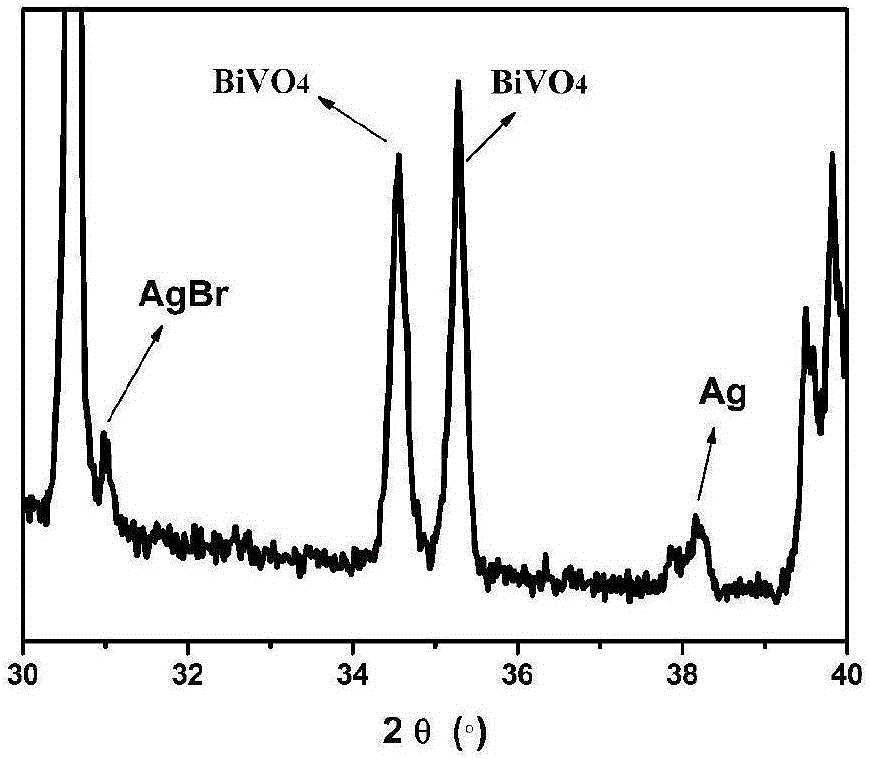
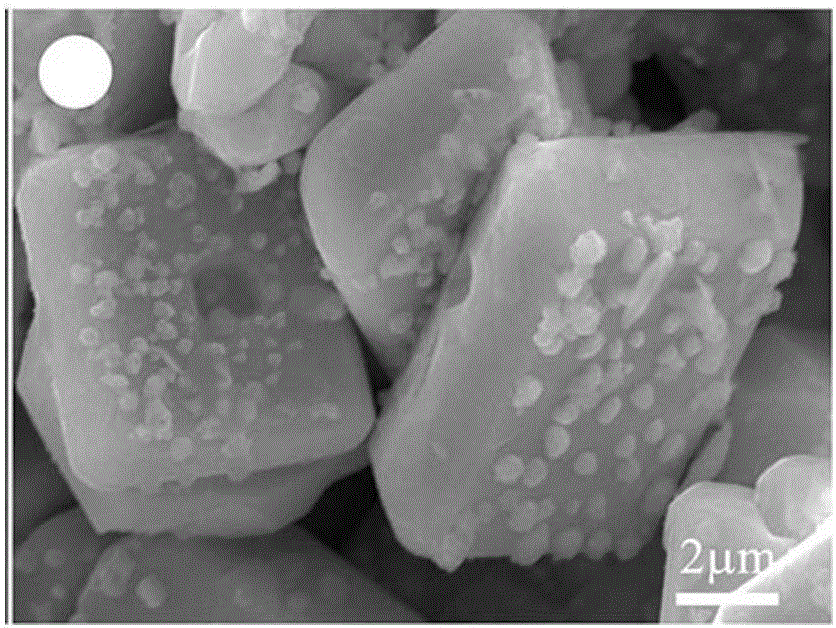
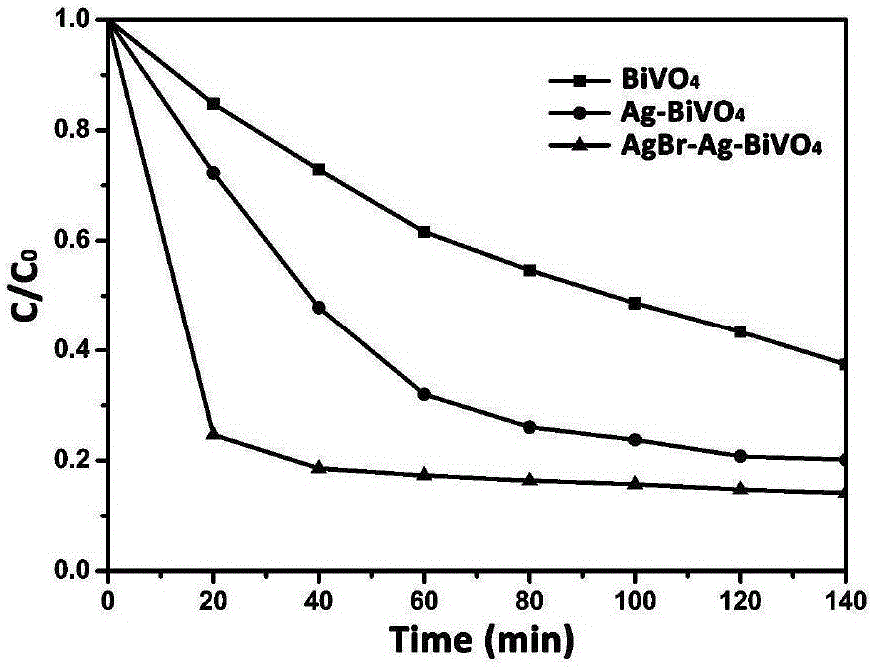
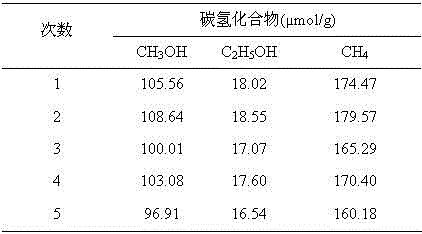
Method for preparing Z-shaped photocatalytic material bismuth vanadate-silver-silver bromide
The invention relates to a method for preparing Z-shaped photocatalytic material bismuth vanadate-silver-silver bromide. The method comprises the following steps: firstly, dispersing bismuth vanadate microcrystals in a water solution, adding a certain quantity of silver bromide, performing stirring till silver bromide is dissolved, and performing irradiation for 3 h by use of a 300 W xenon lamp to enable in-situ reduction of silver ions on the surface of bismuth vanadate to become silver nanoparticles (a faint yellow suspension becomes grey green); then, centrifugally washing obtained sediment for three times to remove impurity ions, and performing drying to obtain bismuth vanadate-silver; and finally, dispersing bismuth vanadate-silver in the water solution, adding ferric nitrate and a potassium bromide solution, enabling reaction for 1 h, and centrifugally washing to obtain a AgBr-Ag-BiVO4 photocatalytic material. The method has the advantages that the operation is simple, reaction conditions are mild, and the preparation period is short. The prepared Z-shaped photocatalytic material can be used for degrading environmental pollutants, such as tetracycline.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of silver halide composite material
InactiveCN102614898AOvercoming technical difficultiesInhibition of agglomerationMolecular sieve catalystsSilver iodideSilver bromide
The invention discloses a preparation method of a silver halide composite material. The silver halide composite material is a silver halide composite which is prepared by loading silver halide on the surface of a carrier with conductive performance, wherein silver halide content occupies 1.0% to 17.5% of weight of the carrier, the silver halide is silver bromide or silver iodide, and the carrier with conductive performance is slice graphite, expansion graphite, graphene, a carbon nano tube, granular activated carbon, zeolite and titanium dioxide. The composite material prepared by the wet method and the precipitation method is capable of enabling ultrafine silver halide nanometer particles to be evenly dispersed on the surface of the carrier with the conductive performance and restrains reuniting on the surface of the silver halide.
Owner:SUN YAT SEN UNIV
Electrochemical method for preparing silver halide photocatalytic material
InactiveCN102335620AHigh catalytic activityEasy to prepareWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsElectrolysisSilver iodide
The invention discloses a method for producing silver halide and a silver halide composite photocatalyst used for (visible) photocatalysis. According to the invention, a quaternary ammonium salt aqueous solution containing different salt brine (or halogen acid) or halogen is subjected to anode electrolysis treatment by a silver electrode to obtain various silver halide materials (silver chloride,silver bromide and silver iodide); a plurality of functional powder materials of oxidized graphene, reduced grapheme, polypyrrole, carbon nanotube and the like are dispersed in the aqueous solution containing different salt brine (or halogen acid), and is subjected to anode electrolysis treatment by the silver electrode to obtain various silver halide composite materials. The ultrasonic effect during the process of electrolysis or after electrolyzing is combined to obtain the composite material powder of silver halide or silver halide. The powder material has good performance of photocatalytic degradation of organic compounds. The method has the advantages of simple process, easy control and easy realization.
Owner:EAST CHINA UNIV OF SCI & TECH
Organic electroluminescent device and production method thereof
InactiveCN104638162AImprove efficiencyImprove luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSilver iodideElectron
The invention discloses an organic electroluminescent device comprising a conductive anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode. The electron injection layer comprises a first doped layer, a second doped layer, a third doped layer, a fourth doped layer and a fifth doped layer stacked in order. The first doped layer, the second doped layer, the third doped layer, the fourth doped layer and the fifth doped layer are made of mixture made by doping a doped material to a base material; the doped material comprises a silver compound and an alkali metal compound; the silver compound is silver iodide, silver sulfide, silver chloride, silver fluoride or silver bromide; the alkali metal compound is lithium fluoride, lithium azide, lithium nitride, cesium fluoride, cesium azide or cesium nitride. The invention further provides a production method of the organic electroluminescent device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Composite light catalyst for quickly and efficiently degrading rhodamine B and preparation method of composite light catalyst
ActiveCN106391066AMild reaction conditionsSimple stepsWater/sewage treatment by irradiationWater treatment compoundsAlcoholNanoparticle
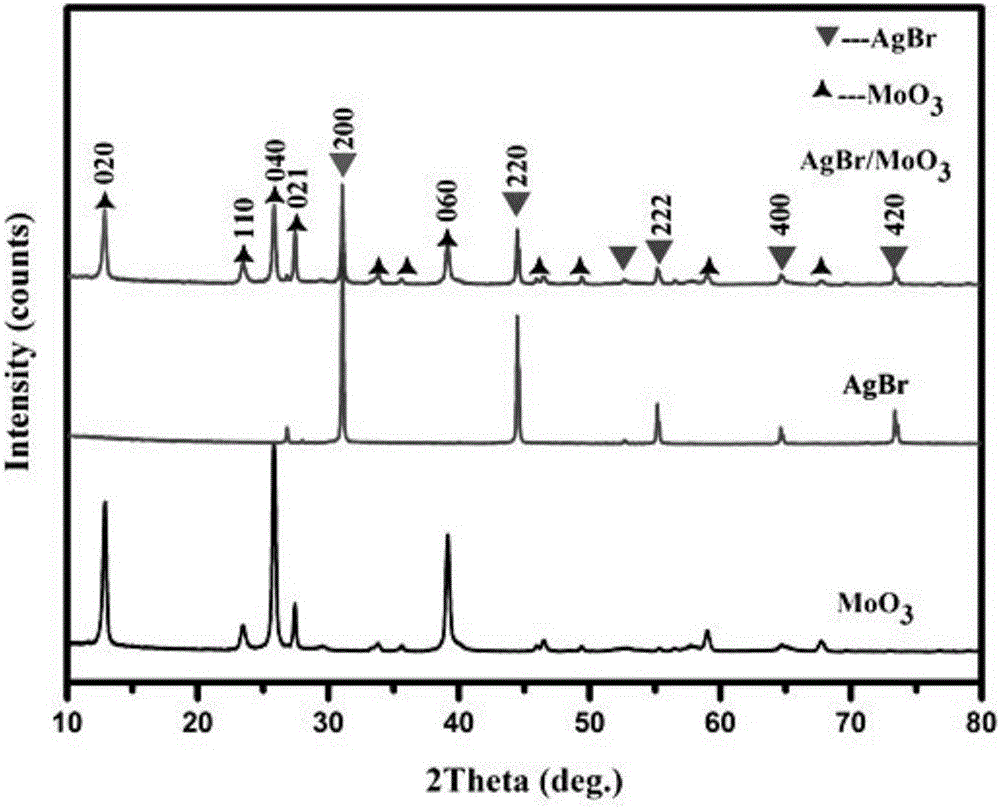
The invention relates to a composite light catalyst for quickly and efficiently degrading rhodamine B and a preparation method of the composite light catalyst. By taking a molybdenum trioxide nanoribbon as a substrate and taking absolute ethyl alcohol as a solvent, an AgBr / MoO3 composite material is synthesized by a sedimentation-settlement method; the preparation method has the advantages of mild reaction conditions, simple steps, greenness, environment friendliness, easily-obtained raw materials and easiness of industrial production. The AgBr / MoO3 composite material prepared by the method is prepared from the strip-shaped molybdenum trioxide nanoribbon serving as the substrate and silver bromide nanoparticles attached to the surface of the substrate. The AgBr / MoO3 photocatalytic material prepared by the method disclosed by the invention can realize quick and efficient degradation of organic matters.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Light-sensitive silver halide photographic film material and radiographic intensifying screen-film combination
InactiveUS6472137B1Increase speedSuitable characteristicX-ray/infra-red processesRadiation applicationsSilver iodideIodide
A light-sensitive silver halide photographic film material has been provided, said film material comprising a transparent support and on both sides thereof at least one light-sensitive emulsion layer having spectrally and chemically sensitized tabular silver halide grains rich in silver bromide, further having silver iodide in an amount of less than 3 mole % based on silver, with two flat parallel {111} crystal faces, said grains accounting for a total projective surface of said parallel crystal faces in said emulsion of at least 50%, further having an average aspect ratio of at least 2:1, a grain thickness of from 0.05 up to 0.15 mum, a site-directing azacyanine compound satisfying the general formulae disclosed herein in an amount of not less than 1x10-4 mole per mole of silver halide coated and one or more J-aggregating spectrally sensitizing dye(s), wherein a molar ratio amount between said site directing compound and said J-aggregating spectrally sensitizing dye(s) is at least 1:6 for a grain coverage of said {111} tabular grains exceeding 50%.A radiographic screen / film combination has also been described comprising said light-sensitive silver halide photographic film material and two supporting or self-supporting X-ray intensifying screens) comprising luminescent phosphors, wherein by contacting the film material with a sandwich of a pair of said intensifying screens and exposing said combination to X-rays, emission of radiation by said luminescent phosphors in the wavelength range for which said material has been made spectrally sensitive provides a black-and-white diagnostic image after processing of said exposed radiographic film material.
Owner:AGFA NV
Preparation method of morphologically homogeneous (111) tabular crystals rich in silver bromide
InactiveUS6087085AGood dimensional stabilityPrevent fadingX-ray/infra-red processesSilver halide emulsionsUF - UltrafiltrationPrecipitation
A method is disclosed for preparing an emulsion having grains rich in silver bromide in the presence of gelatin as a protective colloid in a reaction vessel wherein a yield of more than 250 g of precipitated silver nitrate per liter of reaction vessel mixture is attained, wherein at least 70% of a total projected area of all grains is provided by {111} tabular grains having an average aspect ratio of more than 2:1 and an average thickness of from 0.05 up to 0.30 mu m and wherein a ratio by number of percentage amounts of hexagonal tabular grains to triangular tabular crystals present is more than 10:1, said method comprising following steps: preparing in a reaction vessel a gelatinous dispersion medium containing an initial amount of oxidized gelatin corresponding with less than 50% of a total amount of gelatin used in the said method, and said dispersion medium having a volume of less than 2 liter per 500 g of silver nitrate to be precipitated; precipitating therein silver halide crystal nuclei by double-jet precipitation of an aqueous silver nitrate and an aqueous solution comprising halide ions, wherein less than 10% by weight of a total amount of silver nitrate used is consumed; adding to said reaction vessel gelatin in an amount of more than 50% of a total amount of gelatin used in the said method; growing said silver halide crystal nuclei by further precipitation of silver halide by means of double-jet precipitation of an aqueous silver nitrate solution and an aqueous solution comprising halide ions, wherein more than 90% by weight of a total amount of silver nitrate is consumed, concentrating by ultrafiltration the said reaction mixture volume in the said reaction vessel obtained during precipitation growth steps.
Owner:AGFA HEALTHCARE NV
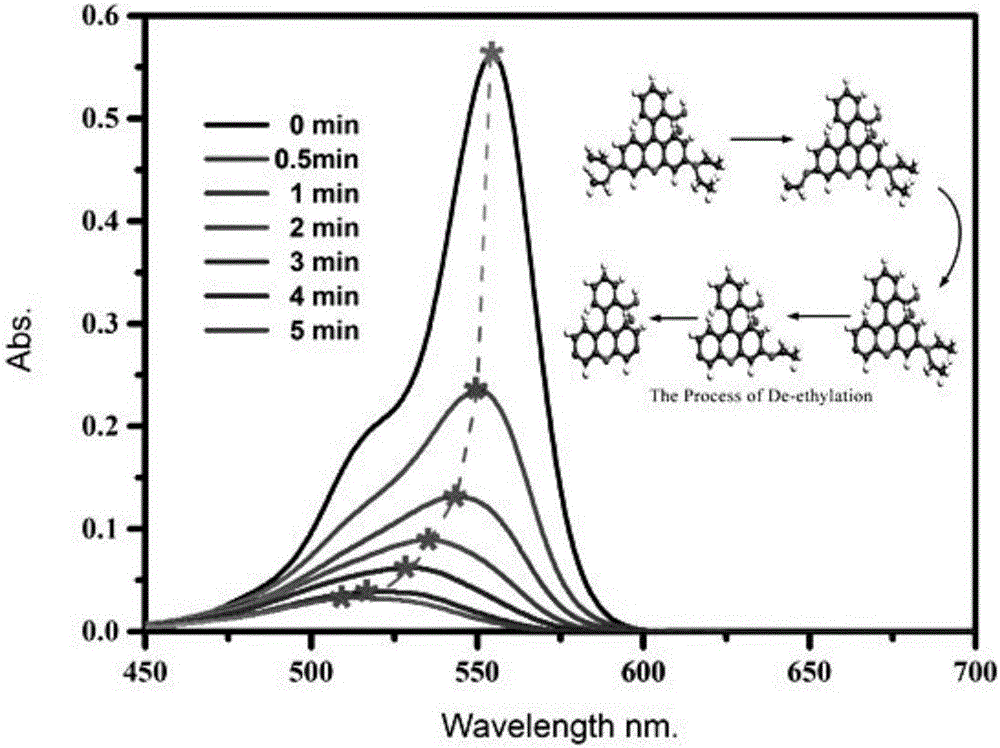
Method for detecting chlorpyrifos based on photoelectrochemical sensor
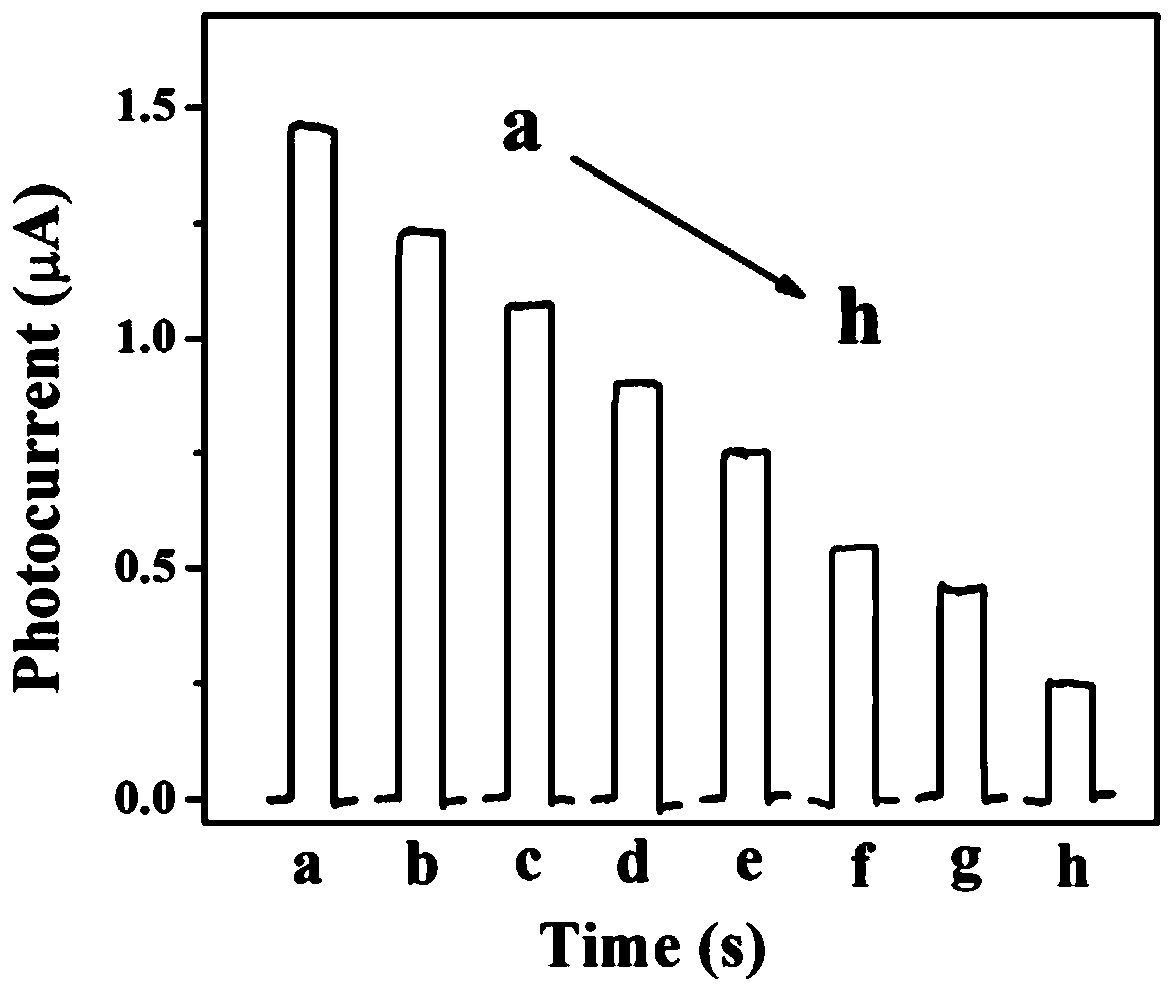
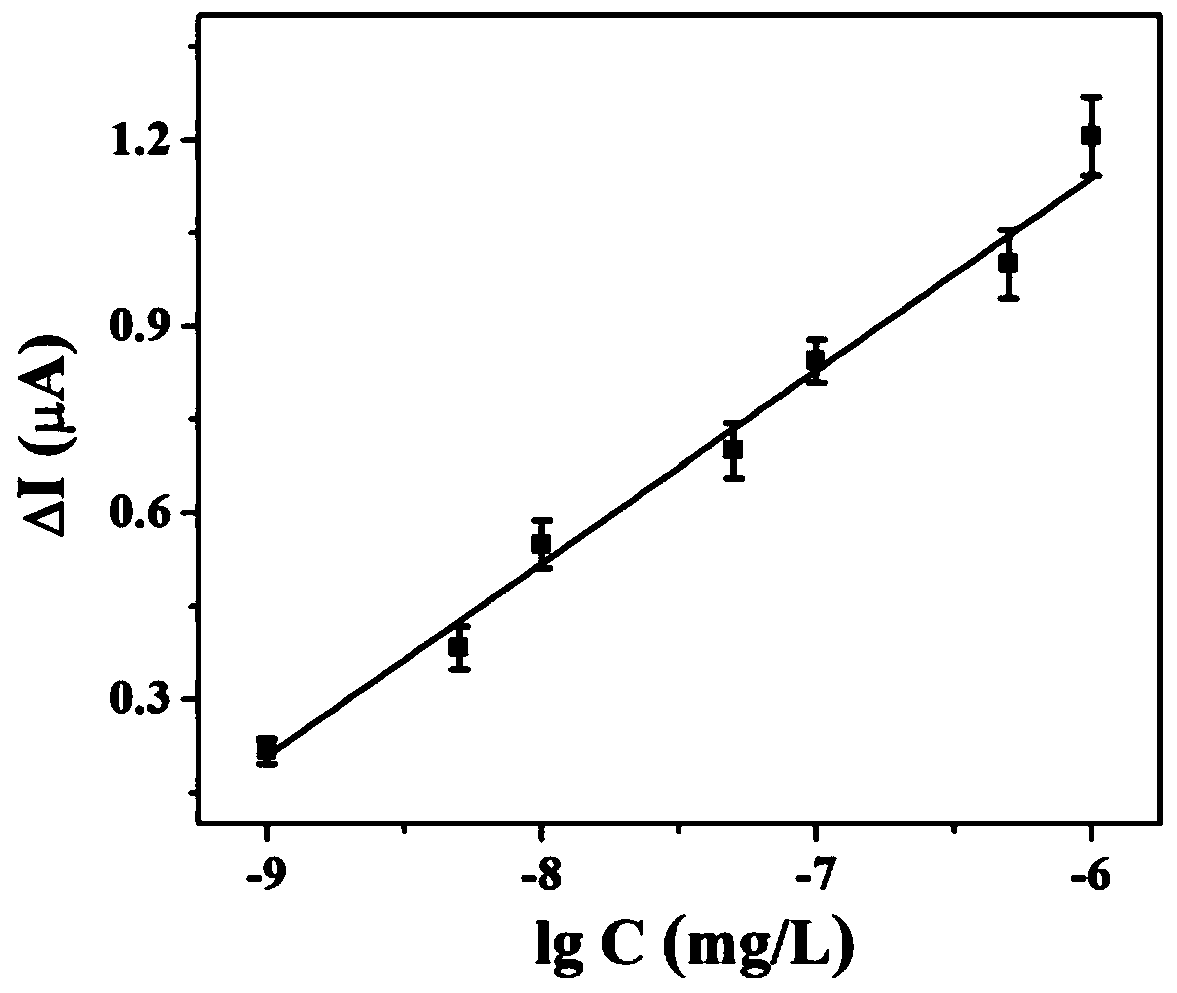
ActiveCN110487868AEasy to operateLow detection limitMaterial analysis by electric/magnetic meansChlorpyrifosSilver bromide
The invention belongs to the field of photoelectrochemical detection, and relates to a method for detecting chlorpyrifos based on a photoelectrochemical sensor. The method comprises the following steps: preparing a titanium carbide-silver bromide nano-composite (Ti3C2-AgBr) by utilizing a wet chemical method; and then modifying the Ti3C2-AgBr nanomaterial on the surface of an electrode of indium tin oxide (ITO) conductive glass, and constructing a sensitive chlorpyrifos photoelectrochemical sensor based on the mechanism of inhibiting the photocurrent of the Ti3C2-AgBr nanomaterial by chlorpyrifos. The detection range of the method is 1.0*10<-9>-1.0*10<-6> mg / L, and the lowest detection limit is 0.33*10<-9> mg / L. The method for detecting the chlorpyrifos is simple to operate, high in sensitivity, good in selectivity and low in detection cost.
Owner:CHANGZHOU UNIV
Silver halide photographic emulsion and silver halide photographic light-sensitive material using the same
InactiveUS20020150847A1Overcome instabilityRaise the ratioSilver halide emulsionsSilver salt compositionsSilver iodideProject area
A silver halide photographic emulsion comprises silver iodochlorobromide tabular grains each having (111) faces as main planes thereof. 70% or more of the total projected area of all the grains contained in the emulsion is occupied by grains each meeting conditions (i) to (iv): (i) a hexagonal tabular grain whose ratio of the length of an edge having the maximum length with respect to the length of an edge having the minimum length, is 2 or less; (ii) an epitaxial junction portion having a silver chloride content of 5 mol % or more and 25 mol % or less, is provided on at least one apex portion of the hexagon; (iii) a silver chloride content thereof is 0.5 mol % or more and 6 mol % or less; and (iv) a silver iodide content thereof is 0.5 mol % or more and 10 mol % or less.
Owner:FUJIFILM CORP +1
Composite silver bromide-bismuth phosphate heterojunction photocatalytic material and preparation method thereof
InactiveCN104607218AShape is easy to controlGood size controlPhysical/chemical process catalystsWater/sewage treatment by irradiationHeterojunctionSilver bromide
The invention discloses a composite silver bromide-bismuth phosphate heterojunction photocatalytic material. Silver bromide nanoparticles are deposited on the bismuth phosphate surface of a micron-sized rod structure, wherein the molar weight of silver bromide is 5.5-14.6% of that of bismuth phosphate. The composite silver bromide-bismuth phosphate heterojunction photocatalytic material has the advantages that agglomeration is not generated; compared with bismuth phosphate and silver bromide, the visible-light catalytic activity is obviously improved; the stability and the performance for repeated use are good; the preparation technology is simple, and the shapes and sizes of materials are easy to control.
Owner:YANAN UNIV
Preparation method and application of purification, sterilization and self-cleaning integrated photosensitive adsorption column material
InactiveCN102847511AStrong self-purification abilityWith self-cleaning functionBiocidePhysical/chemical process catalystsSocial benefitsActivated carbon
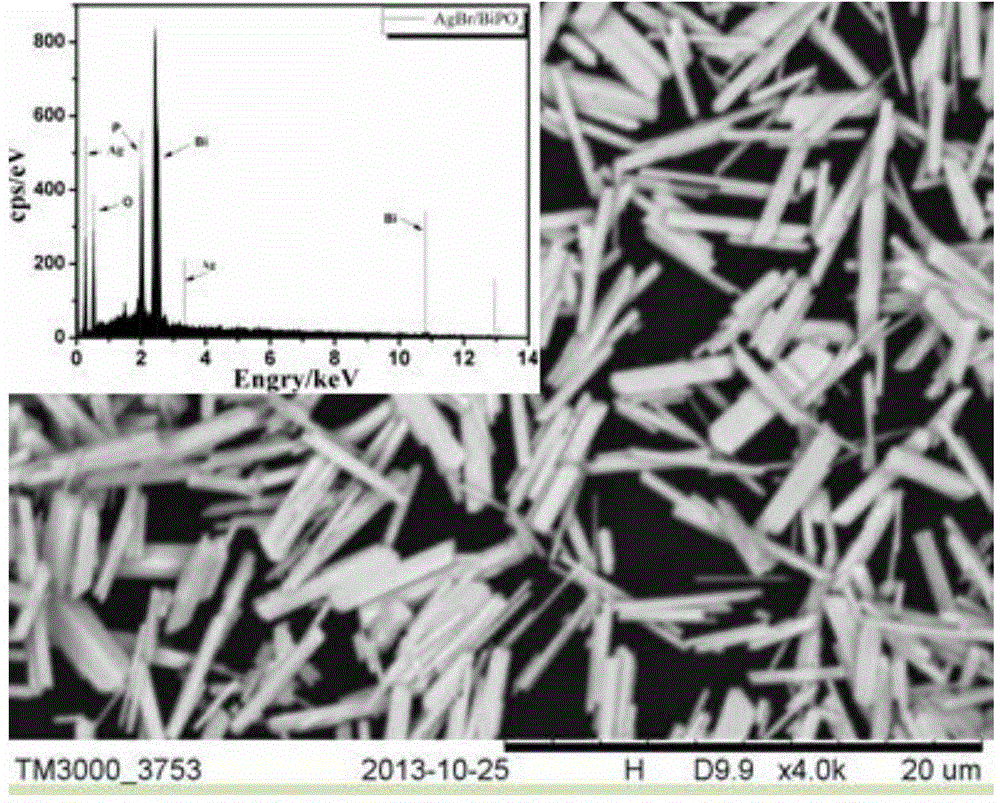
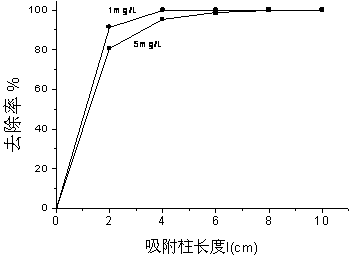
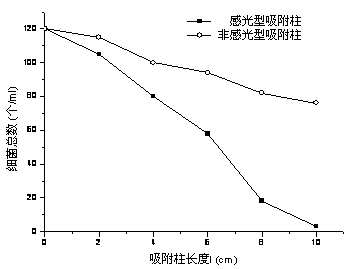
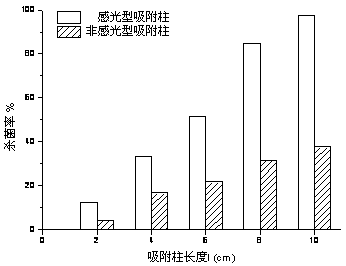
The invention relates to a preparation method and an application of a purification, sterilization and self-cleaning integrated photosensitive adsorption column material. Silver bromide photosensitive agent and titanium dioxide catalyst are loaded onto the surface of a cylindrical carbon aerogel material with stronger adsorption performance than that of active carbon to obtain a novel photosensitive high-efficient adsorption column material with diameter of 1.5cm and length of 2 to 8cm, and the novel photosensitive high-efficient adsorption column material is used as a purification adsorption column for processing tap water. The adsorption function of carbon aerogel for filtering micro contaminants in the water is utilized, a sterilization function of silver (Ag) nano particles formed by radiating a silver bromide (AgBr) sensitiser is used for effectively killing bacteria in the water, the AgBr is used for absorbing the natural light, the photosensitive catalyst which is formed by compounding the AgBr and titanium dioxide is used for catalyzing and decomposing the adsorbed contaminants and obtaining a self-cleaning function, and the three functions are ingeniously combined into a whole. The photosensitive adsorption column material is simple in preparation process, low in production cost and wide in economic and social benefit.
Owner:赵思沉
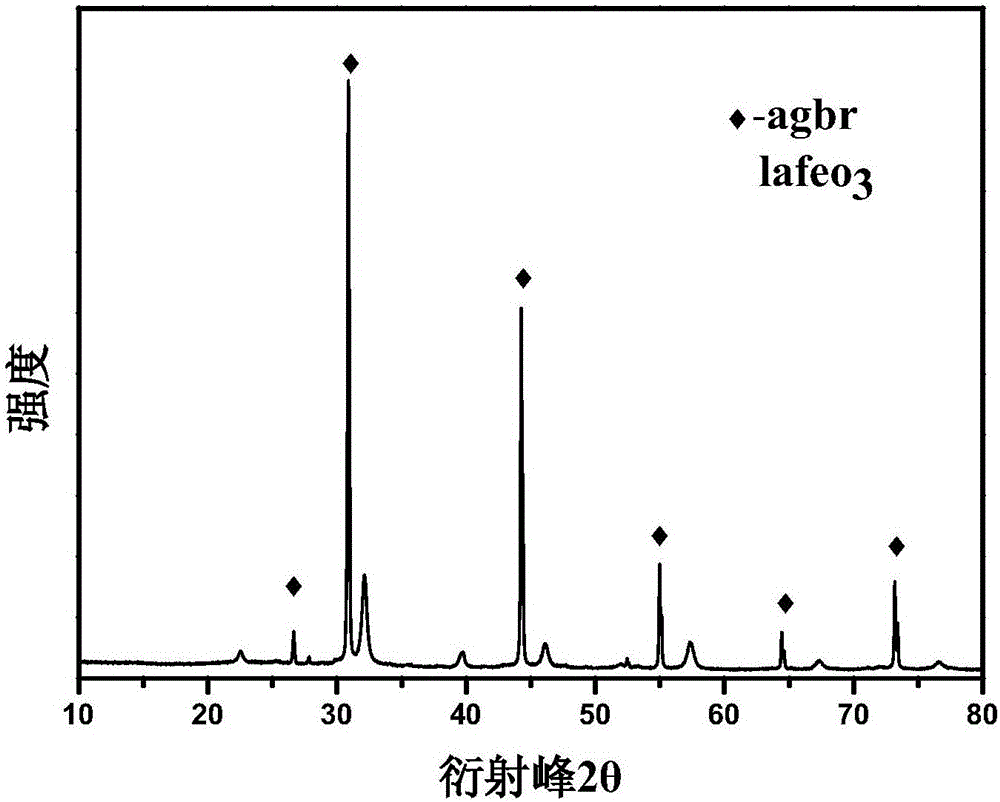
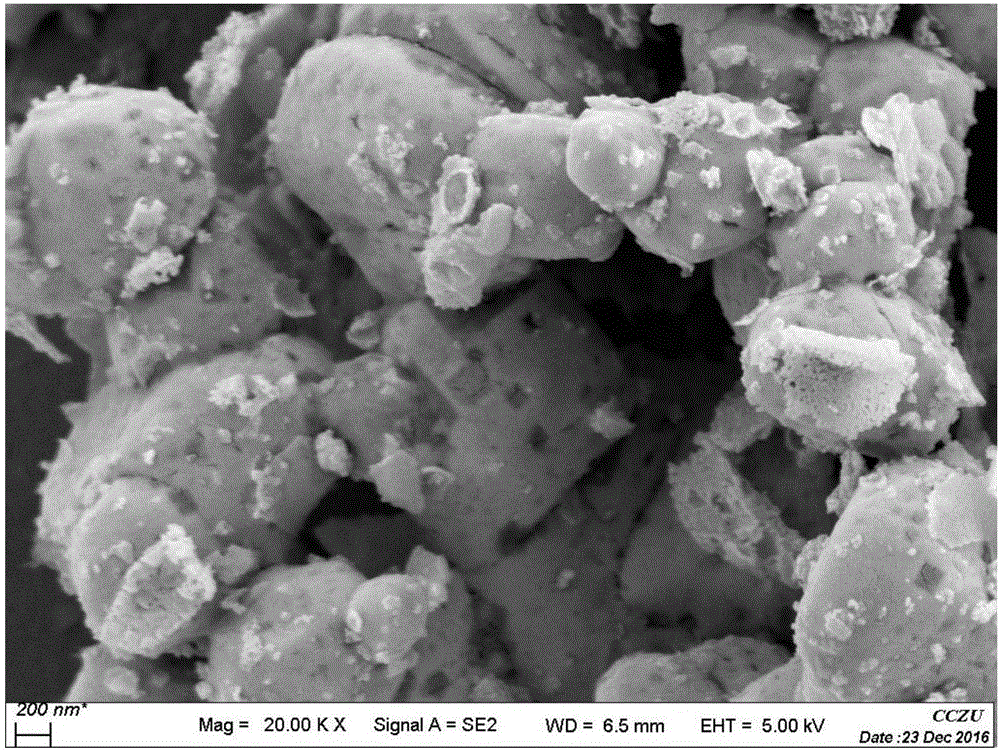
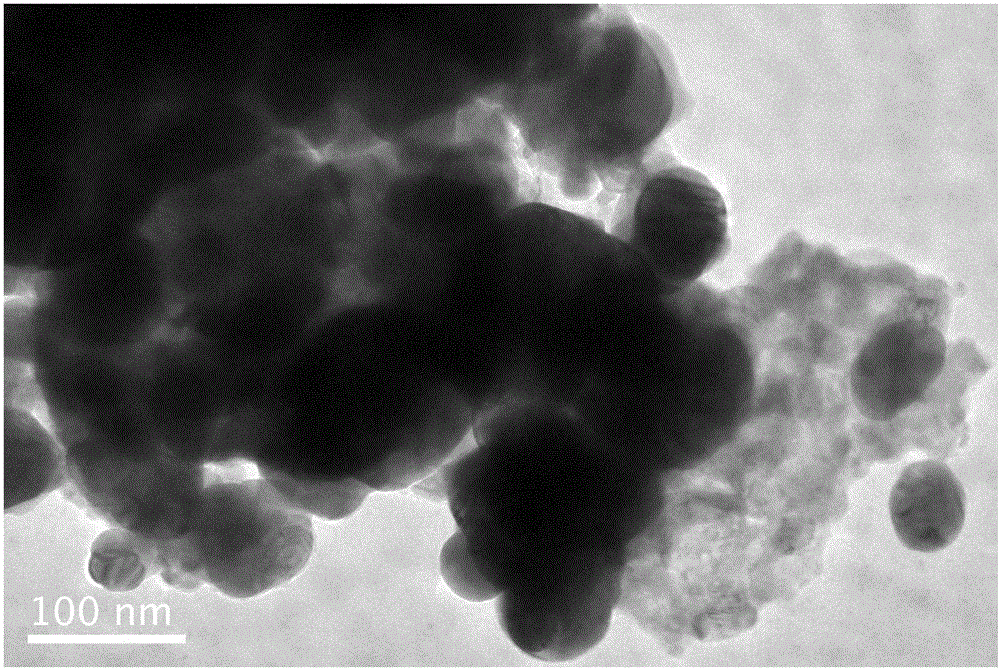
Preparation method of lanthanum ferrite doped silver bromide compound photocatalyst
ActiveCN106807411ANo pollution in the processHigh catalytic efficiencyPhysical/chemical process catalystsWater/sewage treatment by irradiationSilver bromideLanthanum
The invention relates to a preparation method of a lanthanum ferrite doped silver bromide compound photocatalyst. The preparation method comprises the following contents: preparation of lanthanum ferrite and preparation of the lanthanum ferrite doped silver bromide compound photocatalyst. The preparation method has the beneficial effects that the preparation method is relatively simple and convenient, the preparation conditions are easy to control, and the prepared lanthanum ferrite doped silver bromide compound photocatalyst is a green and environmentally-friendly high-performance catalyst, is free of pollution and high in catalytic efficiency and has certain application values.
Owner:CHANGZHOU UNIV
Silver halide photographic emulsion and silver halide photographic lightsensitive material using the same
InactiveUS20030190561A1Great advantageIncrease contentSilver halide emulsionsSilver salt compositionsSilver bromideSilver particles
A silver halide photographic emulsion comprising grains, wherein 50% or more (numerical ratio) of all the grains are occupied by tabular grains with epitaxial junction meeting the requirements (i) to (v): (i) silver iodochlorobromide grains having (111) faces as main planes and having two parallel twin planes, (ii) an equivalent circle diameter of 3.0 mum or more and an aspect ratio of 8 or more, (iii) each of host tabular grains has six silver halide epitaxial junction portions selectively in apex portions thereof, (iv) at least one of the silver halide epitaxial junction portions has at least one dislocation line, and (v) a spacing between the two parallel twin planes of 0.012 mum or less.
Owner:FUJIFILM CORP +1
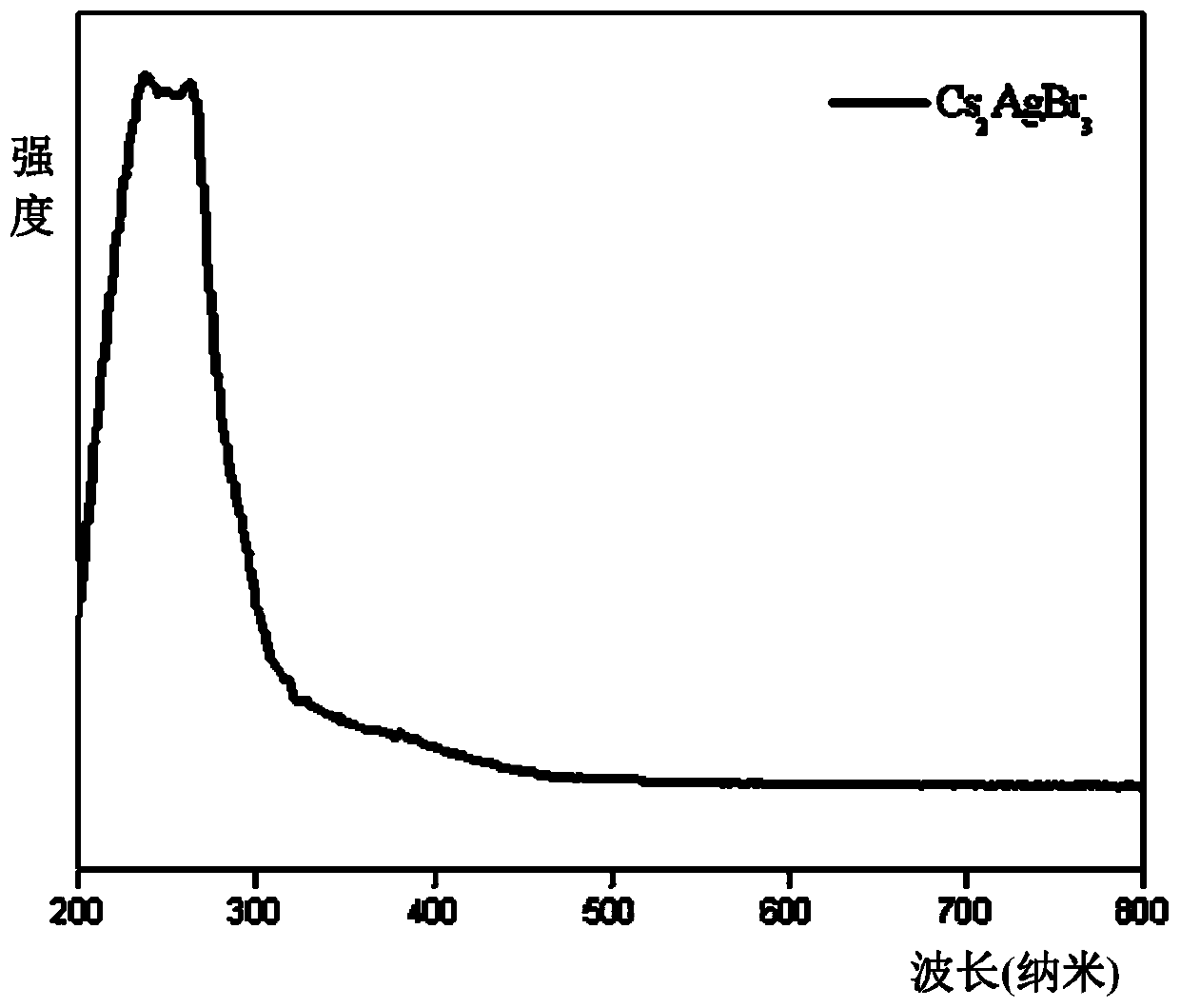
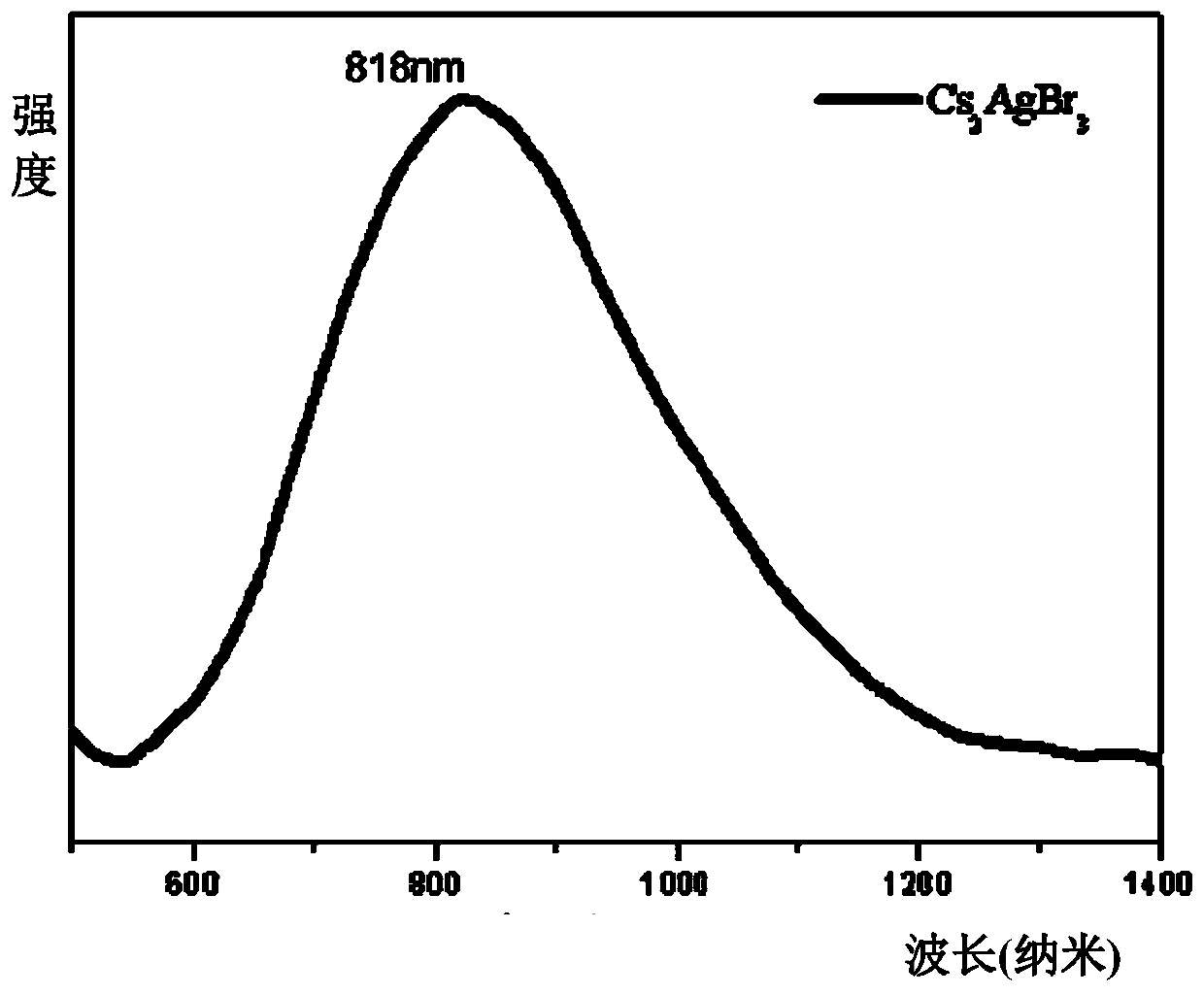
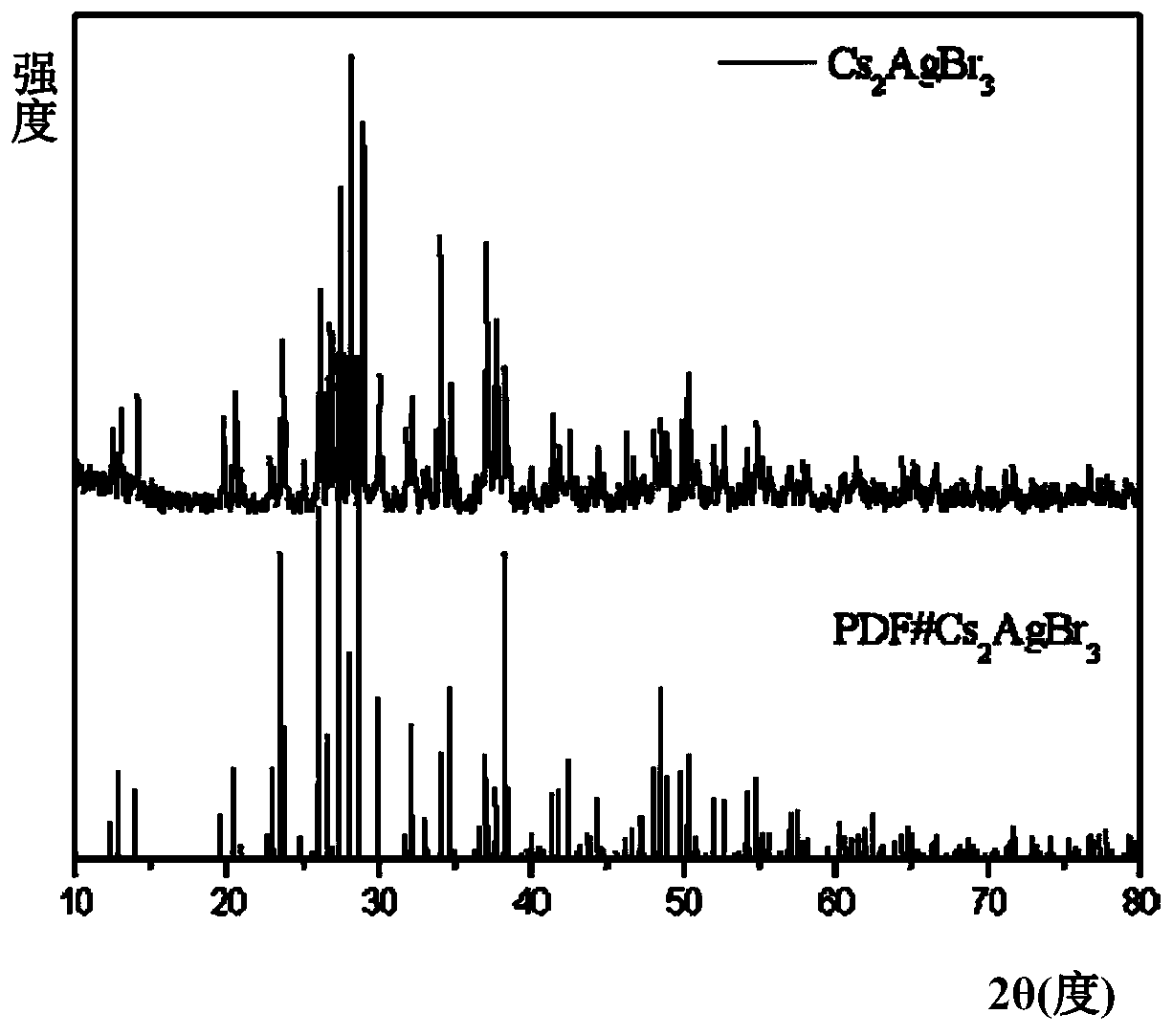
Method for efficient synthesis of Cs2AgBr3 non-lead all-inorganic perovskite
InactiveCN110862103AShort timeEasy to operateLuminescent compositionsSilve compoundsFluorescenceSilver bromide
Belonging to the technical field of preparation of semiconductor nanomaterials, the invention discloses a method for efficient synthesis of Cs2AgBr3 non-lead all-inorganic perovskite. The method includes: firstly mixing cesium bromide and silver bromide according to a molar ratio of 2:1, then adding tri-n-octyl phosphorus, performing grinding, gradually hardening the mixture from fluffy faint yellow powder, and attaching the mixture to the wall of a container, gradually turning the faint yellow powder to white powder along with the grinding, performing monitoring by a 302nm ultraviolet lamp inthe grinding process, and stopping the action when the brightness of the product does not increase any more; and subjecting the obtained product to heat treatment in a vacuum oven at 80-300DEG C for3h, and then conducting freezing treatment at a temperature ranging from -10DEG C to -50DEG C for 1h-3h, thus obtaining the Cs2AgBr3 non-lead all-inorganic perovskite with improved fluorescence yield.The method provided by the invention has the advantages of short time, easy operation and suitability for large-scale production.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com