Method for efficient synthesis of Cs2AgBr3 non-lead all-inorganic perovskite
A technology of inorganic calcium and titanium ore, applied in inorganic chemistry, chemical instruments and methods, silver compounds, etc., can solve the problems of unavailability, long reaction time and cooling time, resistance to Cs, etc., and achieves simple operation and is suitable for large-scale production. , the effect of short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
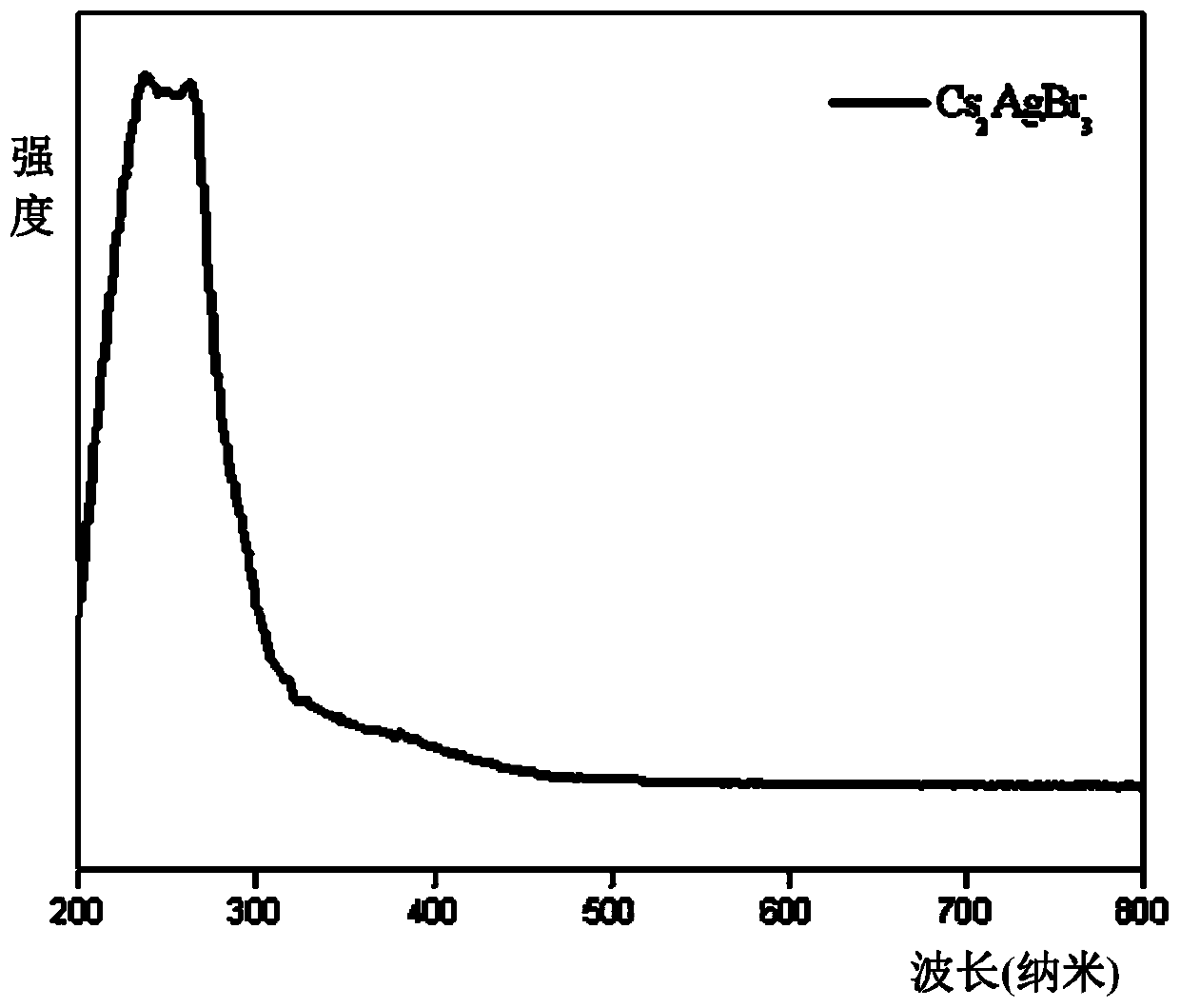
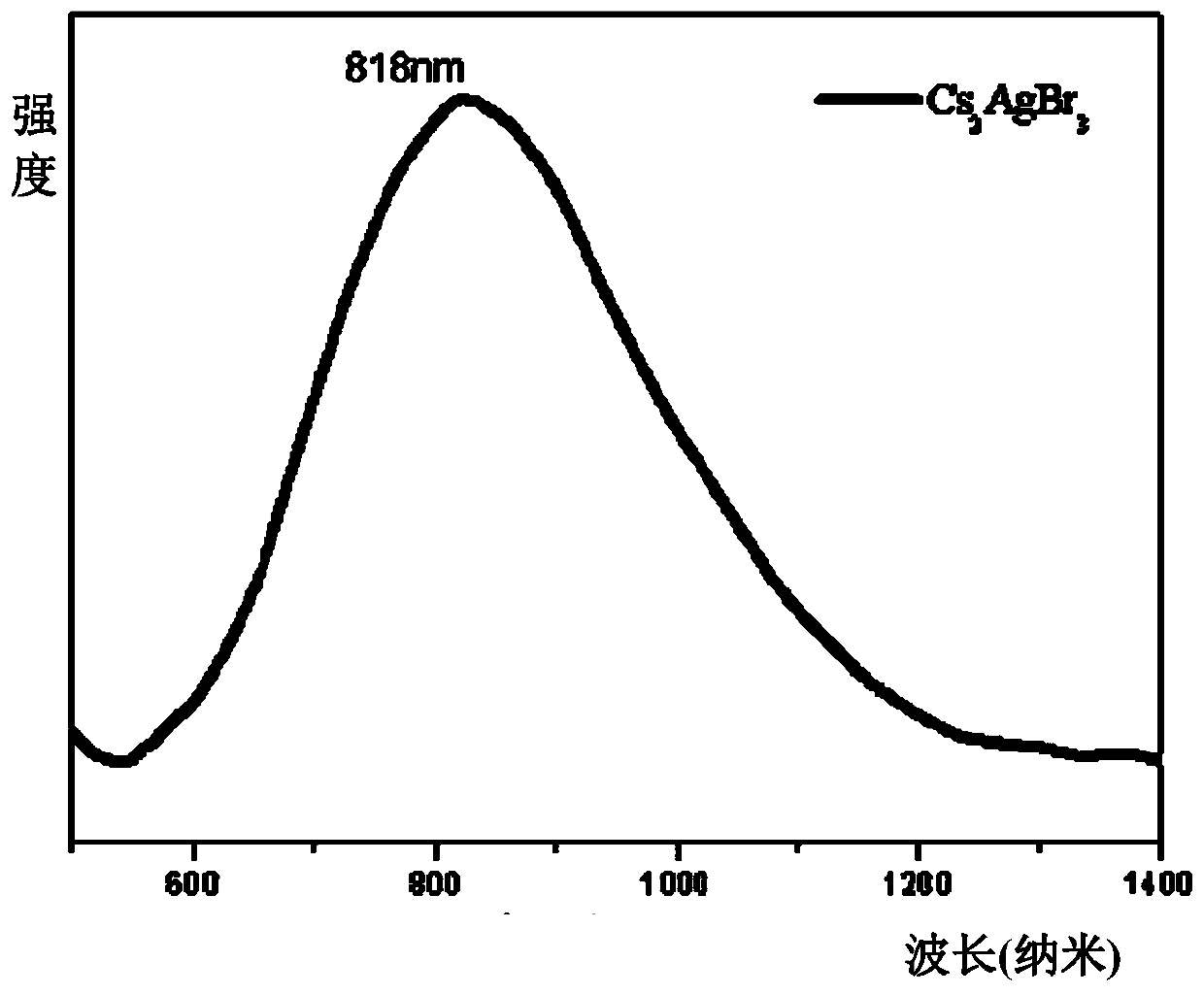
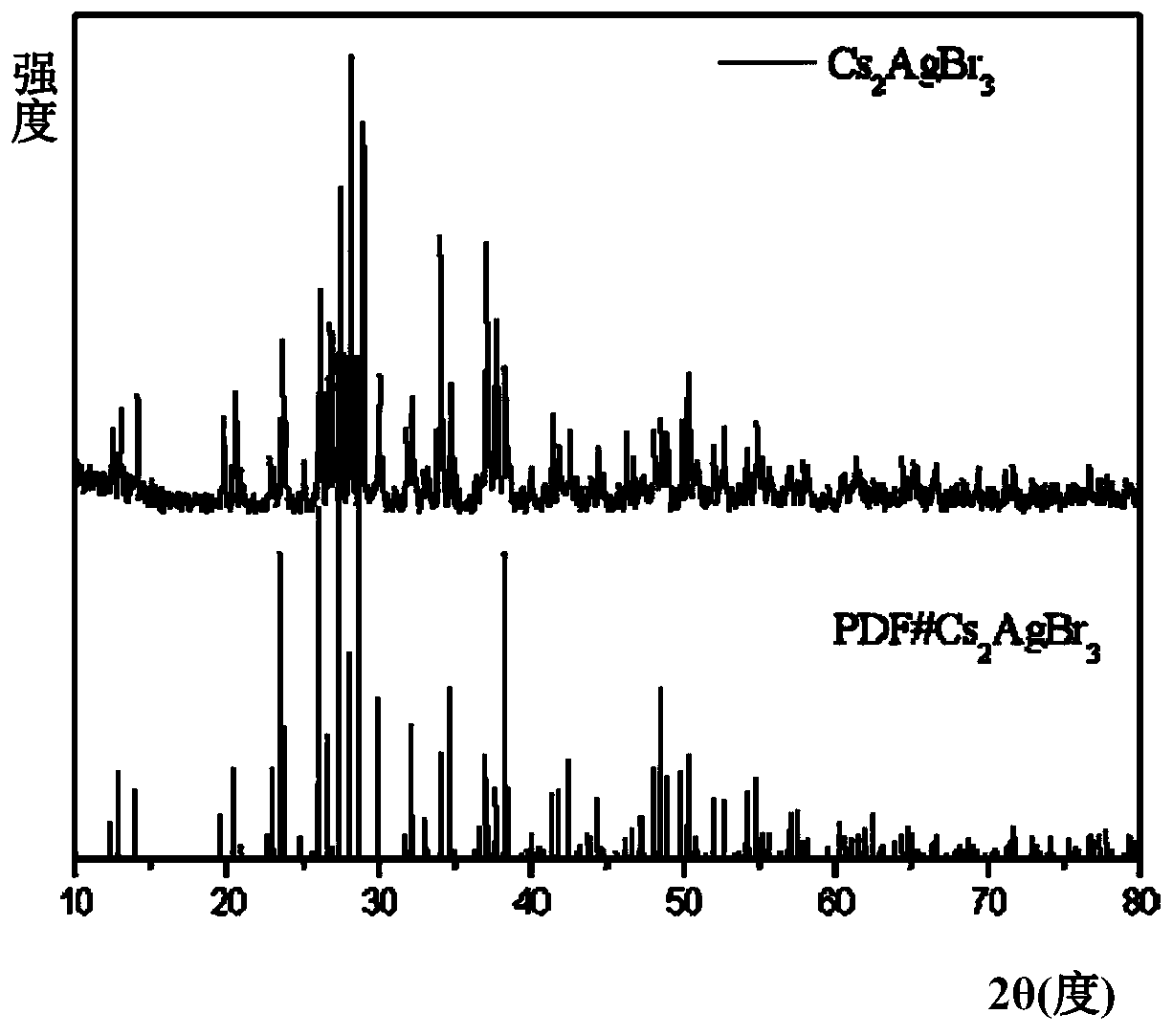
[0018] Put 2mmol cesium bromide, 1mmol silver bromide, 60ul tri-n-octyl phosphorus, and 25 agate balls with a diameter of 6mm into a 25ml agate jar. Grinding for 2 hours, the mixture gradually hardened from a fluffy light yellow powder and adhered to the wall of the agate jar. It was irradiated with a 302nm ultraviolet lamp, and it was found that the fluorescence brightness of the product did not continue to increase. The product was heat-treated in a vacuum oven at 150°C for 2 hours. The white Cs obtained after heat treatment 2 AgBr 3 The product was frozen at -30°C for 2 hours, and the product was subjected to solid absorption analysis and fluorescence testing. The absorption spectrum was as follows: figure 1 As shown, the emission spectrum is shown as figure 2 As shown, the fluorescence efficiency is 40.3%, and the XRD pattern of the product is as follows image 3 shown by image 3 It can be seen that the Cs prepared according to the method of Example 1 2 AgBr 3 is p...
Embodiment 2
[0019] Embodiment 2: The consumption of the tri-n-octylphosphine in the example 1 is changed to 30uL, 50uL, 80uL, 100uL respectively by 60uL, other conditions are unchanged, the fluorescence quantum efficiency of the product is respectively 25.6%, 36.7%, 33.9%, 33.4%, so the optimal dosage of tri-n-octylphosphine is 60uL.
Embodiment 3
[0020] Embodiment 3: The temperature of the heat treatment in the vacuum oven in Example 1 was changed from 150°C to 80°C, 100°C, 200°C, and 300°C, and the fluorescent quantum efficiencies of the products were 30.2%, 36.2%, 34.1%, and 33.7%, respectively, Therefore, the optimal heat treatment temperature is 150°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com