Method for preparing Z-shaped photocatalytic material bismuth vanadate-silver-silver bromide
A photocatalytic material, a technology of bismuth vanadate, applied in the field of photocatalysis, can solve the problems of low utilization efficiency of sunlight, hindering application, low photon quantum efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
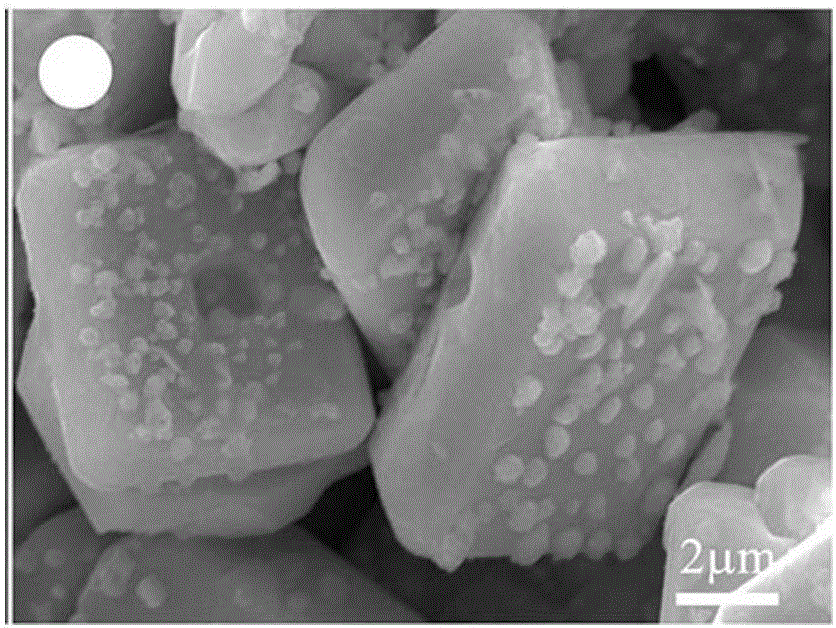
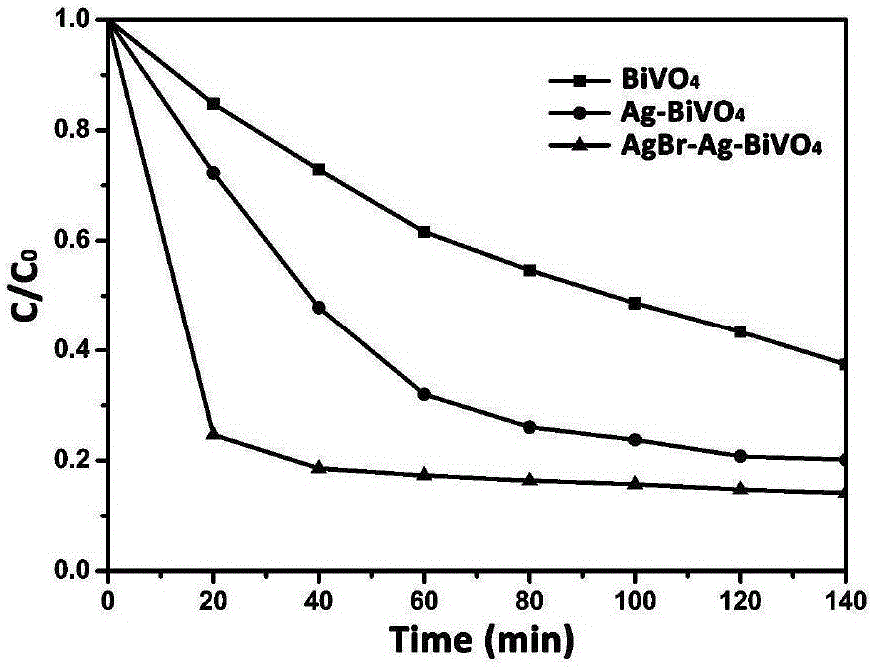
[0014] Disperse bismuth vanadate microcrystals into the aqueous solution, add a certain amount of silver nitrate (calculate the amount of silver nitrate added so that the weight percentage of silver in bismuth vanadate-silver is 3%), and stir until the silver nitrate dissolves Finally, irradiate with a 300 W xenon lamp for 3 hours, so that the silver ions are in situ reduced to silver nanoparticles on the surface of bismuth vanadate (suspension changes from light yellow to gray-green), and the obtained precipitate is centrifuged and washed 3 times to remove impurities. ions, dried to obtain bismuth vanadate-silver; bismuth vanadate-silver was dispersed in the aqueous solution, adding ferric nitrate and potassium bromide solution with a molar ratio of 1:1, reacted for 1 hour, centrifuged and washed to obtain vanadic acid Bismuth-silver-silver bromide photocatalytic material.
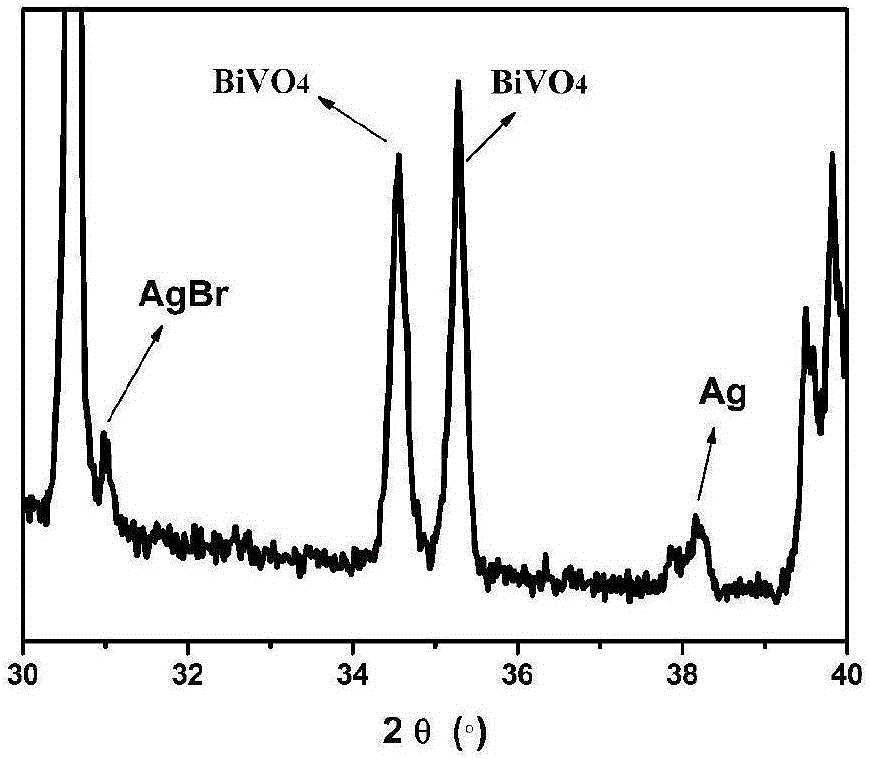
[0015] figure 1 Be that embodiment 1 obtains the X-ray diffraction pattern of bismuth vanadate-silver...
Embodiment 2
[0017] Disperse bismuth vanadate microcrystals into the aqueous solution, add a certain amount of silver nitrate (calculate the amount of silver nitrate added so that the weight percentage of silver in bismuth vanadate-silver is 5%), and stir until the silver nitrate dissolves Finally, irradiate with a 300 W xenon lamp for 3 hours, so that the silver ions are in situ reduced to silver nanoparticles on the surface of bismuth vanadate (suspension changes from light yellow to gray-green), and the obtained precipitate is centrifuged and washed 3 times to remove impurities. ions, dried to obtain bismuth vanadate-silver; bismuth vanadate-silver was dispersed in the aqueous solution, adding ferric nitrate and potassium bromide solution with a molar ratio of 1:1, reacted for 1 hour, centrifuged and washed to obtain vanadic acid Bismuth-silver-silver bromide photocatalytic material.
Embodiment 3
[0019] Disperse bismuth vanadate microcrystals into the aqueous solution, add a certain amount of silver nitrate (calculate the amount of silver nitrate added so that the weight percentage of silver in bismuth vanadate-silver is 8%), and stir until the silver nitrate dissolves Finally, irradiate with a 300 W xenon lamp for 3 hours, so that the silver ions are in situ reduced to silver nanoparticles on the surface of bismuth vanadate (suspension changes from light yellow to gray-green), and the obtained precipitate is centrifuged and washed 3 times to remove impurities. ions, dried to obtain bismuth vanadate-silver; bismuth vanadate-silver was dispersed in the aqueous solution, adding ferric nitrate and potassium bromide solution with a molar ratio of 1:1, reacted for 1 hour, centrifuged and washed to obtain vanadic acid Bismuth-silver-silver bromide photocatalytic material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com