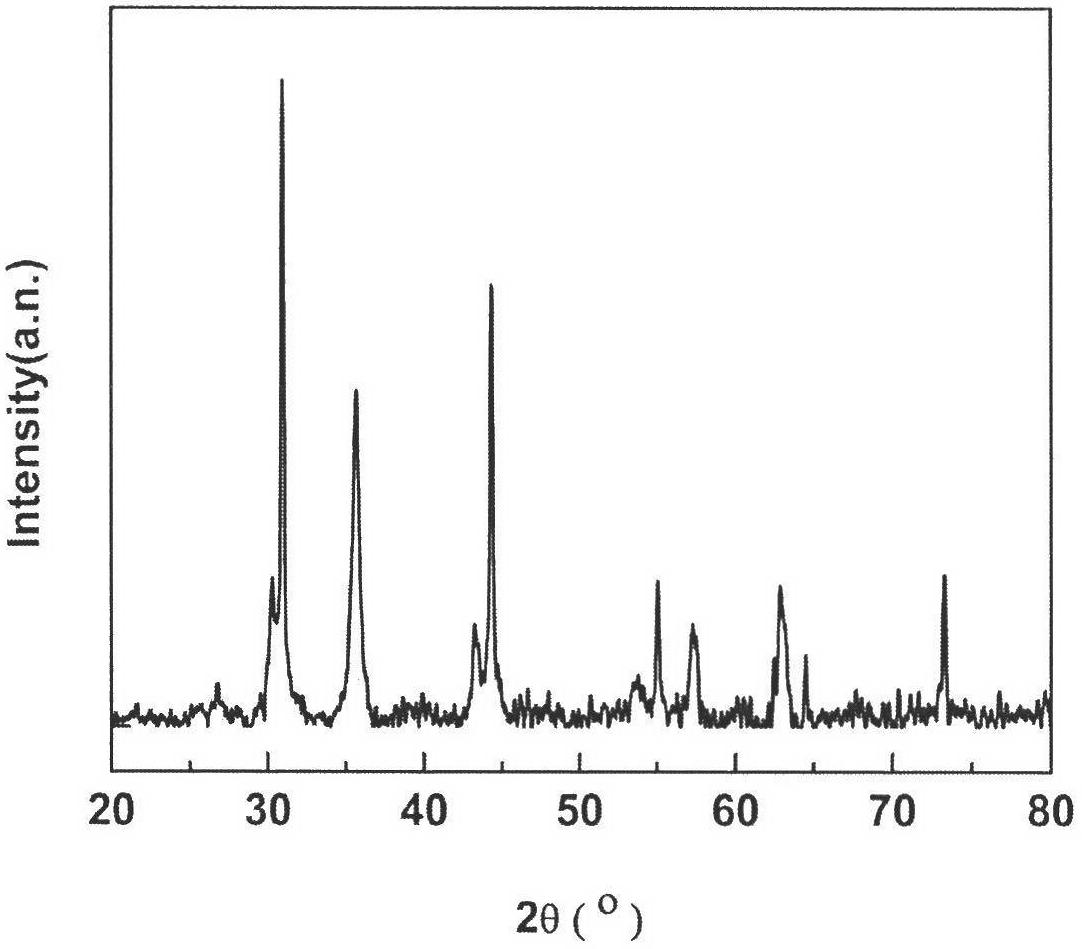
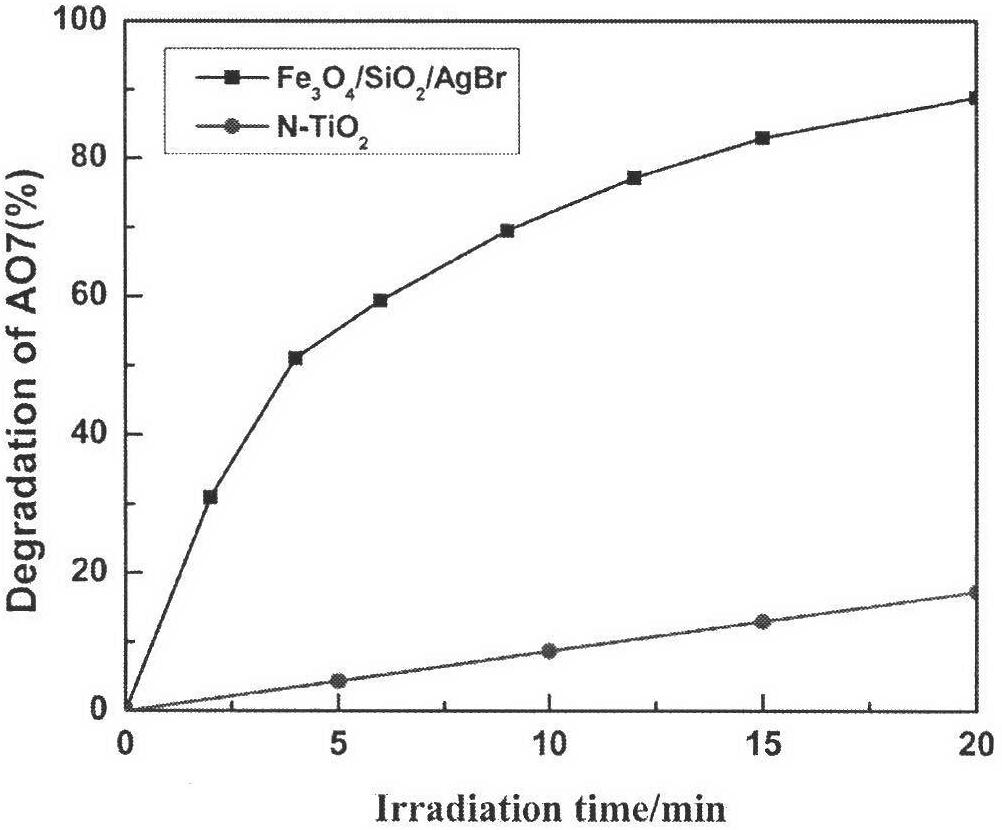
Magnetically supported silver bromide photochemical catalysis material and preparation method thereof
A photocatalytic material, silver bromide technology, applied in the field of photocatalysis, can solve problems such as difficult to recycle and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve ferric chloride in ethylene glycol, add anhydrous sodium acetate and 24g / L polyethylene glycol, stir the mixture vigorously for 30 minutes, and then carry out hydrothermal reaction. After the reaction, wash three times with absolute ethanol and secondary water respectively, and disperse in absolute ethanol; take the above-mentioned ethanol solution, add absolute ethanol, secondary water, ultrasonically disperse, and slowly add dropwise ethyl orthosilicate ( The molar ratio of ethyl orthosilicate to ferric oxide is 1:1), quickly add ammonia water dropwise, and keep ultrasonic reaction at room temperature. After the end, wash with absolute ethanol and secondary water several times, magnetically separate, and disperse in water; under ultrasonic and mechanical stirring conditions, take the above-mentioned product, add silver nitrate and ammonia water quickly, and slowly add potassium bromide (bromide) The molar ratio of potassium to ferric oxide is 1:1) aqueous sol...
Embodiment 2
[0025] Dissolve ferric chloride in ethylene glycol, add anhydrous sodium acetate and 12g / L polyethylene glycol, stir the mixture vigorously for 30 minutes, and then carry out hydrothermal reaction. After the reaction, wash three times with absolute ethanol and secondary water respectively, and disperse in absolute ethanol; take the above-mentioned ethanol solution, add absolute ethanol, secondary water, ultrasonically disperse, and slowly add dropwise ethyl orthosilicate ( The molar ratio of ethyl orthosilicate to ferric oxide is 1:1), quickly add ammonia water dropwise, and keep ultrasonic reaction at room temperature. After the end, wash with absolute ethanol and secondary water several times, magnetically separate, and disperse in water; under ultrasonic and mechanical stirring conditions, take the above-mentioned product, add silver nitrate and ammonia water quickly, and slowly add potassium bromide (bromide) The molar ratio of potassium to ferric oxide is 1:1) aqueous sol...
Embodiment 3
[0027] Dissolve ferric chloride in ethylene glycol, add anhydrous sodium acetate and 24g / L polyethylene glycol, stir the mixture vigorously for 30 minutes, and then carry out hydrothermal reaction. After the reaction, wash three times with absolute ethanol and secondary water respectively, and disperse in absolute ethanol; take the above-mentioned ethanol solution, add absolute ethanol, secondary water, ultrasonically disperse, and slowly add dropwise ethyl orthosilicate ( The molar ratio of ethyl orthosilicate to ferroferric oxide is 1.2:1), quickly add ammonia water dropwise, and keep ultrasonic reaction at room temperature. After the end, wash with absolute ethanol and secondary water several times, magnetically separate, and disperse in water; under ultrasonic and mechanical stirring conditions, take the above-mentioned product, add silver nitrate and ammonia water quickly, and slowly add potassium bromide (bromide) The molar ratio of potassium to ferric oxide is 1:1) aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com