Preparation method of silver halide composite material
A composite material and silver halide technology, applied in chemical instruments and methods, molecular sieve catalysts, chemical/physical processes, etc., can solve problems such as low efficiency and low quantum yield, and achieve improved efficiency, enhanced response, and light absorption The effect of strength enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
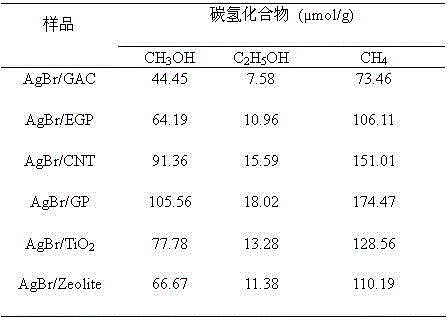
[0033] Reality Embodiment 1: Preparation of AgBr composite material
[0034] 1 g of GP, EGP, GO, CNT, GAC, Zeolite, TiO 2 The solid powder was added to 100 ml of 0.01 mol / L cetyltrimethylammonium bromide (CTAB) aqueous solution, ultrasonically dispersed for 30 minutes, and then stirred with a magnetic stirrer for 30 minutes to obtain a uniformly distributed suspension A. Take 0.0012 mol of AgNO 3 Add to 2.3 ml NH 4 OH (25 wt. % NH 3 ) to obtain mixed solution B. Then the B mixed solution was quickly added to the suspension A, and after magnetic stirring at room temperature for 12 h, the resulting precipitate was separated by a high-speed centrifuge and washed with ethanol for 5 times, and then dried at 75 °C. Then calcined at different temperatures of 200°C, 400°C, 500°C and 700°C for 3 hours to obtain 20.0% AgBr / GP, AgBr / EGP, AgBr / GO, AgBr / CNT, AgBr / GAC, AgBr / Zeolite, AgBr / TiO 2 composite material. Prepare 1.0%, 10.0%, 30.0% and 50.0% AgBr / GP, AgBr / EGP, AgBr by contr...
Embodiment 2
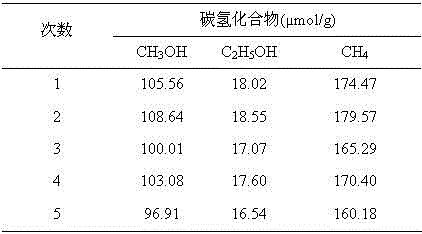
[0035] Example 2: AgX / GP Visible Light Catalytic CO 2 preparation of hydrocarbons
[0036] Visible light catalyzed CO 2 The reaction device for preparing hydrocarbons adopts a closed cylindrical stainless steel reactor. The stainless steel reactor has its own valve to facilitate the control of gas input and discharge. In order to facilitate the illumination, there is a sealable circular glass window with optical filter above the stainless steel reactor. A 500 W medium-pressure xenon light source was used as the visible light source to provide visible light of λ≥420 nm. Take 0.5 g of synthesized 1.0%, 10.0%, 20.0%, 30.0% and 50.0% (wt.AgBr / GP) AgBr / GP composite samples and disperse them in 100 ml of 0.2 mol / L KHCO 3 A suspension was formed in the solution, and the pH of the solution was adjusted to 8.5 with NaOH. Pure CO was continuously fed into the suspension through the control valve in the stainless steel reactor before the illumination started. 2 gas (purity 9...
Embodiment 3
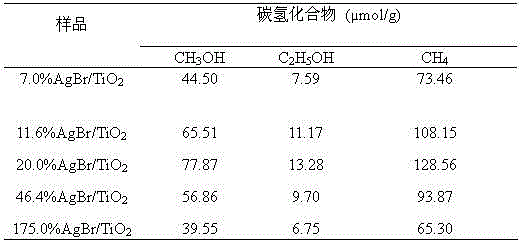
[0039] Example 3: AgX / TiO 2 Visible light catalyzed CO 2 preparation of hydrocarbons
[0040] The stainless steel reactor described in Example 2 was used as the reaction device, and a 500 W medium-pressure xenon lamp light source was used to provide the visible light source with λ≥420 nm of visible light. 7.0%, 11.6%, 20.0%, 46.4% and 175.0% (wt.AgBr / wt.TiO 2 ) a series of AgBr / TiO 2 Nanocatalyst sample, dispersed in 100ml of 0.2 mol / L KHCO 3 A suspension was formed in the solution, and the pH of the solution was adjusted to 8.5 with NaOH. Pure CO was continuously fed into the suspension through the control valve in the stainless steel reactor before the illumination started. 2 gas (99.99% purity) for 30 min to remove oxygen from the solution. Then close the control valve and continue to introduce CO 2 The gas maintains the pressure in the reactor at about 7.5 MPa. Turn on the xenon lamp light source under magnetic stirring, and continue to irradiate for 5h....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com