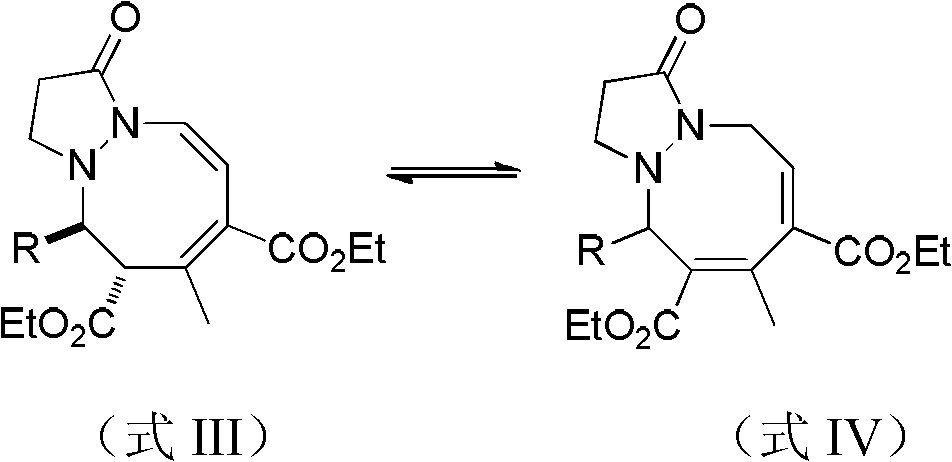
1,2-oxinane pyrazolidone compounds and preparation method and applications thereof
A compound, alkyl technology, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
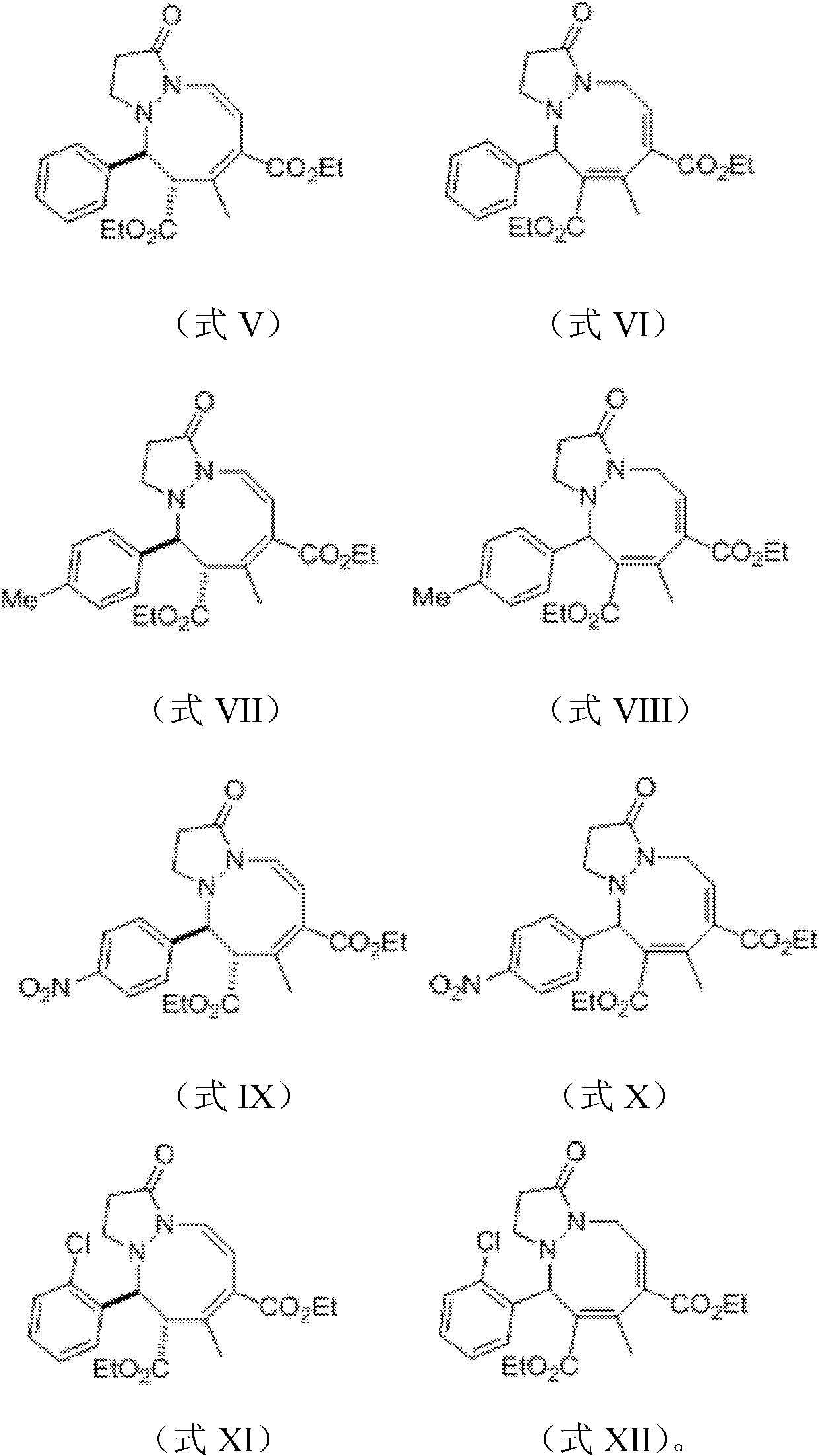
[0033] Synthesis of 1,2-azacyclooctan pyrazolidinone compounds shown in embodiment 1, formulas V and VI
[0034]
[0035] 0.0218g of compound 1-(benzylidene)-3-oxodihydropyrazole ylide (0.125mmol) shown in formula I, 4mL of dichloromethane, 1mL of benzene and 0.0280g of compound 2 shown in formula II, Ethyl 3-butadienoate (0.250mmol) was put into a 15mL Shrek tube dried in an oven, and the phosphine catalyst tricyclohexylphosphine 0.025mmol was added and mixed for cycloaddition reaction. In this reaction system, 1-( The molar ratio of benzylidene)-3-oxodihydropyrazole ylide to ethyl 2,3-butadienoate is 1:2.0, and tricyclohexylphosphine accounts for 1-(benzylidene)- The molar percentage of 3-oxodihydropyrazole ylide was 20%, stirred at 0°C for 120 hours, concentrated the reaction solution with a rotary evaporator, and passed the column (ethyl acetate:petroleum ether=10:1, v / v), obtain 40.3mg product, be the mixture of compound shown in formula V and VI, yield 81%, calculat...
Embodiment 2
[0040] Synthesis of 1,2-azacyclooctan pyrazolidinone compounds shown in embodiment 2, formula (VII) and (VIII)
[0041]
[0042] Compound 1-[(4-methylphenyl)methylene]-3-oxodihydropyrazole ylide (0.125mmol) shown in 0.0235g formula I, 3mL dichloromethane, 2mL benzene and 0.0308g The compound 2,3-butadienoic acid ethyl ester (0.275mmol) shown in the formula II was put into the 15mL Shrek tube dried in an oven, and the phosphine catalyst trimethylphosphine 0.0125mmol was added and mixed to carry out the cycloaddition reaction. In the reaction system, the molar ratio of 1-[(4-methylphenyl)methylene]-3-oxodihydropyrazole ylide to 2,3-butadienoic acid ethyl ester is 1: 2.2, Trimethylphosphine accounts for 10% of the molar percentage of 1-[(4-methylphenyl)methylene]-3-oxodihydropyrazole ylide, stirred at -5°C for 130 hours, and used The reaction solution was concentrated by a rotary evaporator and passed through the column (ethyl acetate:petroleum ether=10:1, v / v) to obtain 33.0...
Embodiment 3
[0047] Synthesis of 1,2-azacyclooctanopyrazolidinone compounds shown in embodiment 3, formula (IX) and (X)
[0048]
[0049] Compound 1-[(4-nitrophenyl)methylene]-3-oxodihydropyrazole ylide (0.125mmol) shown in 0.0274g formula I, 5mL dichloromethane and 0.0336g formula II Compound 2, ethyl 3-butadienoate (0.300mmol) was put into the 15mL Shrek tube dried in an oven, and the phosphine catalyst tributylphosphine 0.00625mmol was added and mixed to carry out the cycloaddition reaction. In the reaction system , The molar ratio of 1-[(4-nitrophenyl)methylene]-3-oxodihydropyrazole ylide to 2,3-butadienoic acid ethyl ester is 1:2.4, tributyl Phosphine accounts for 5% mole percent of 1-[(4-nitrophenyl)methylene]-3-oxodihydropyrazole ylide, stirred at 25°C for 80 hours, and concentrated with a rotary evaporator The reaction solution was passed through the column (ethyl acetate:petroleum ether=10:1, v / v) to obtain 29.0 mg of product, which was a mixture of compounds represented by fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com