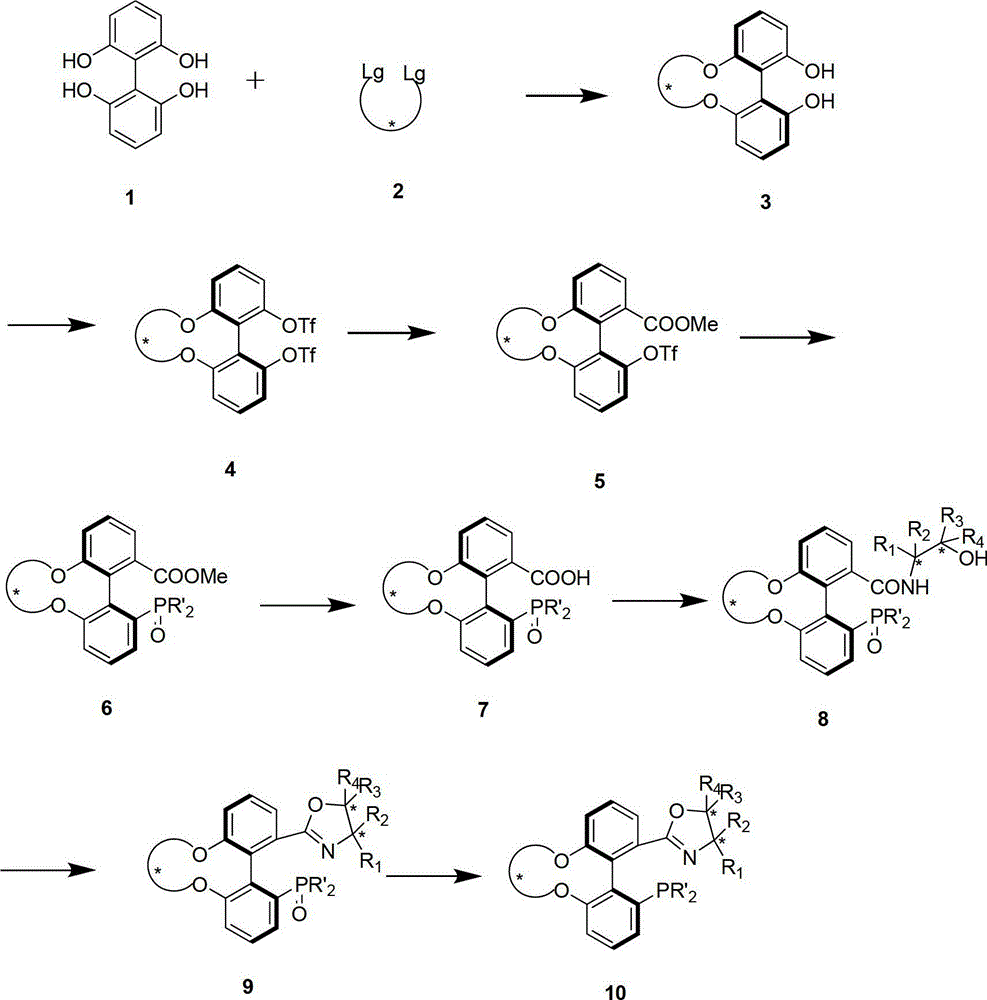
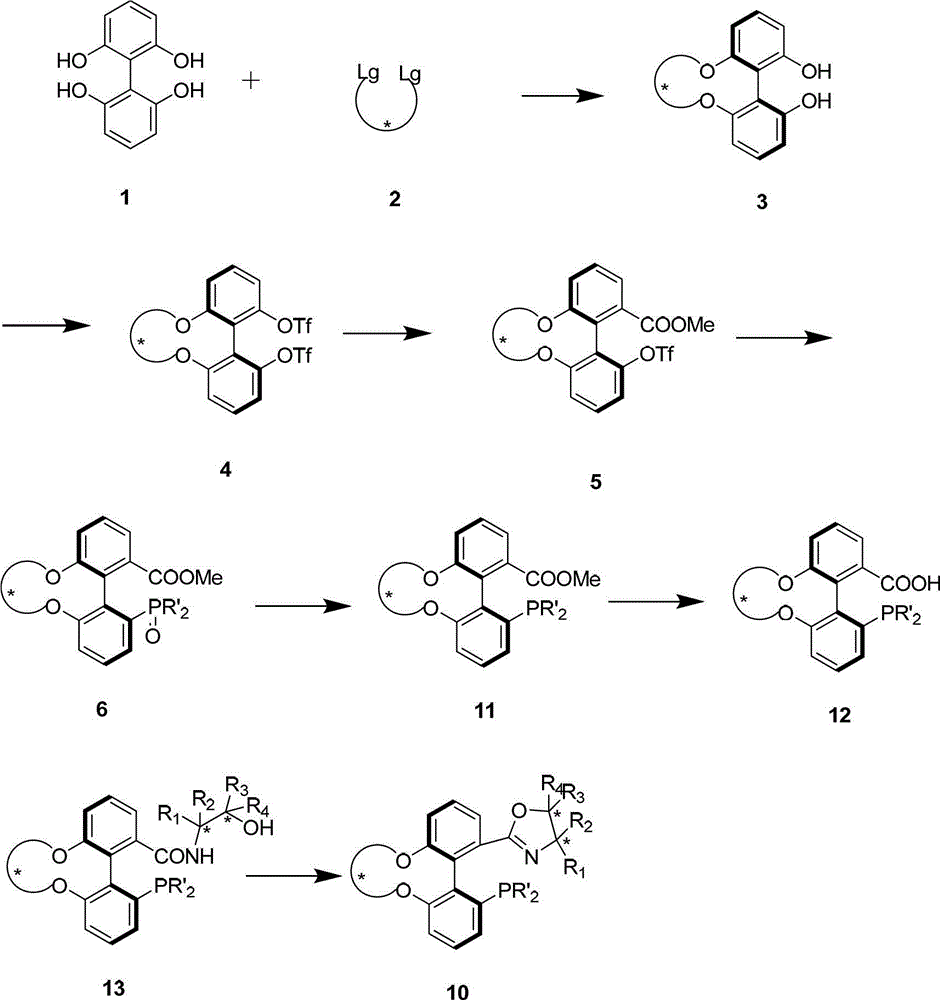
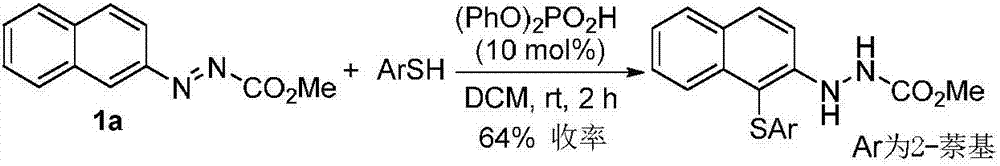
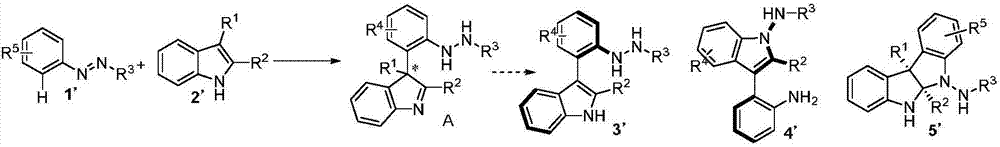
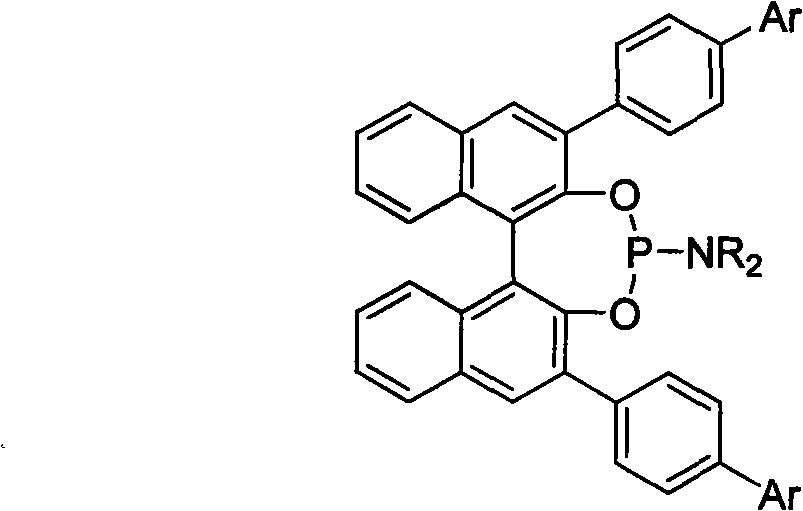
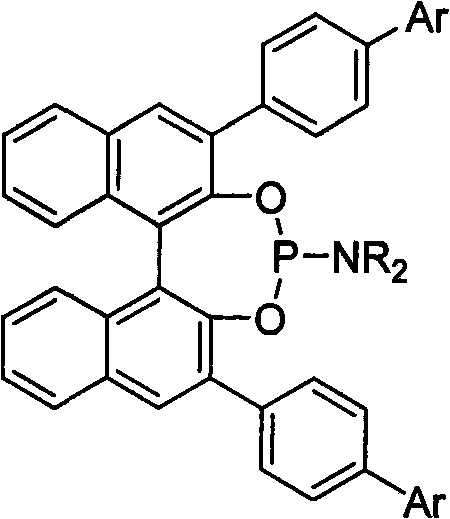
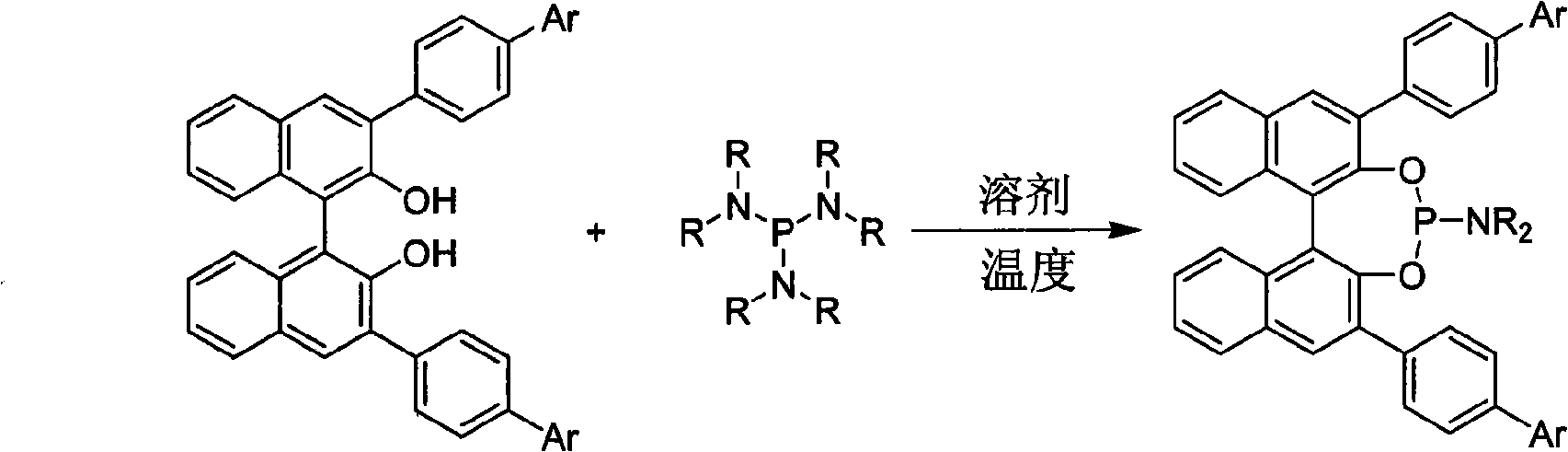
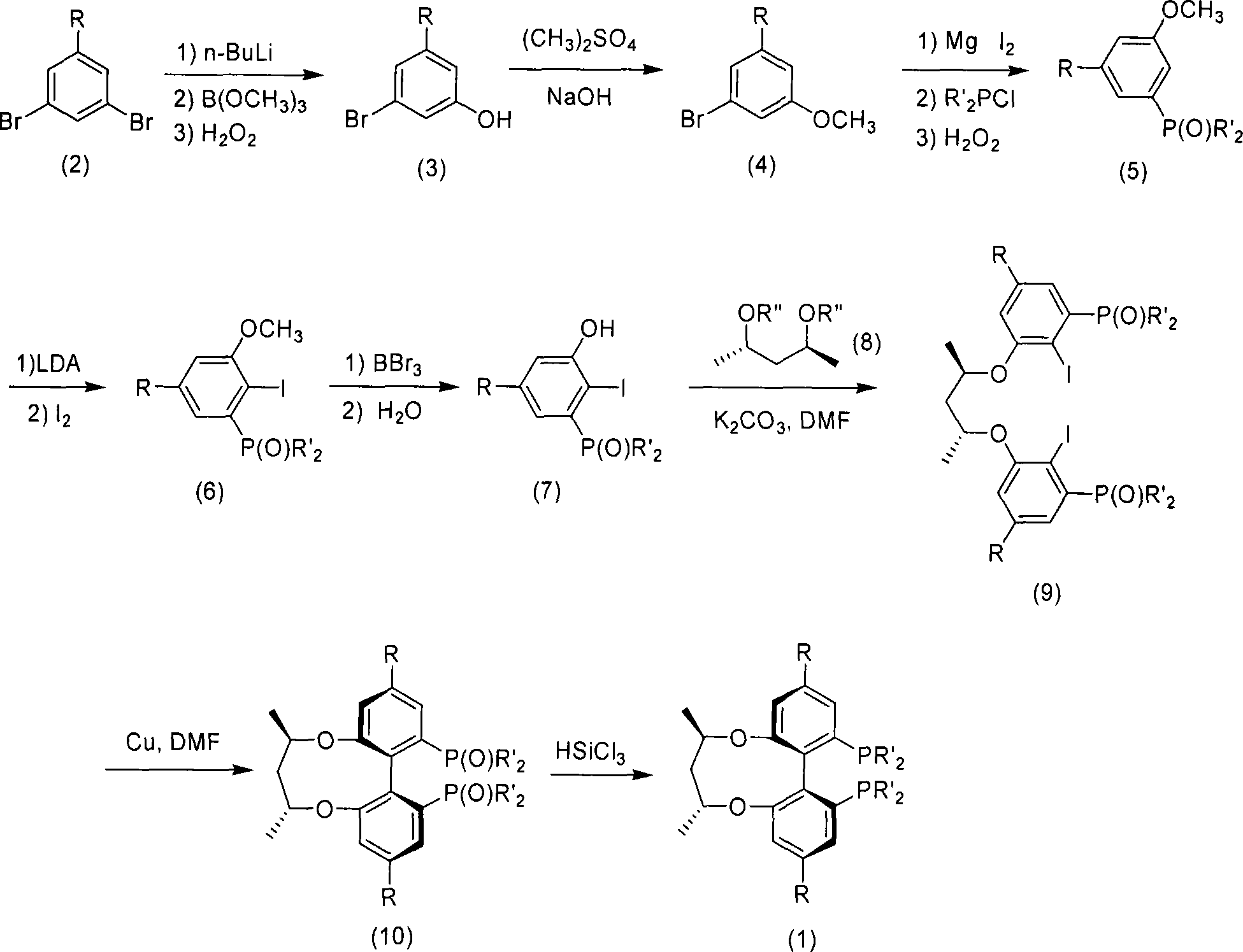
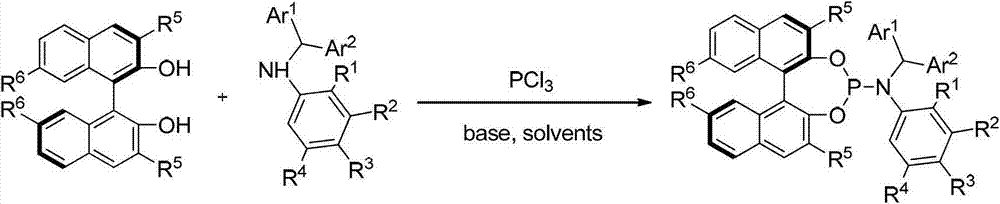
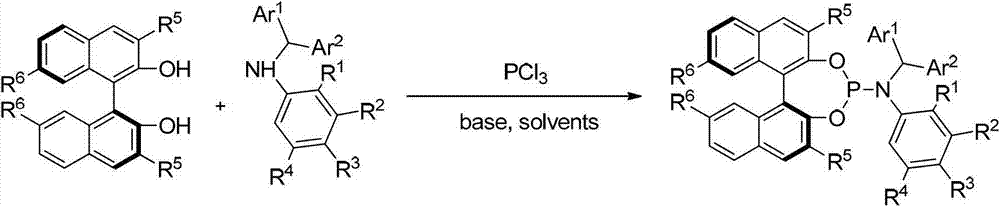
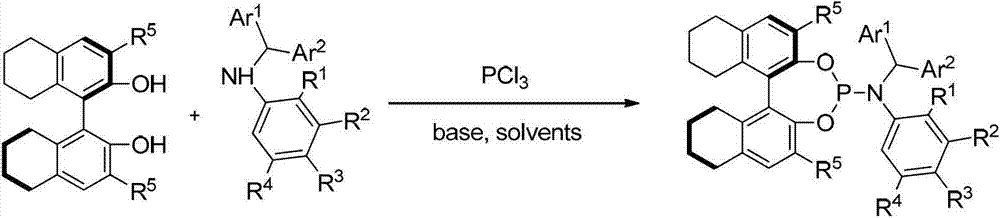
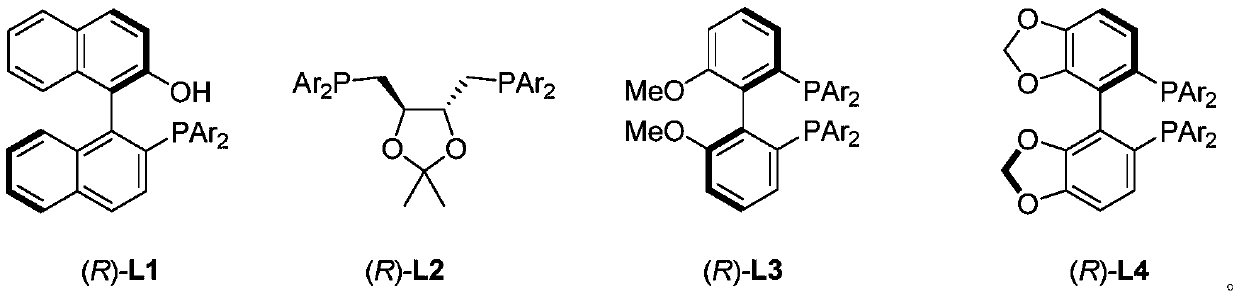
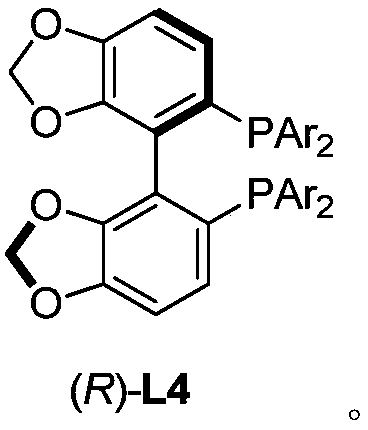
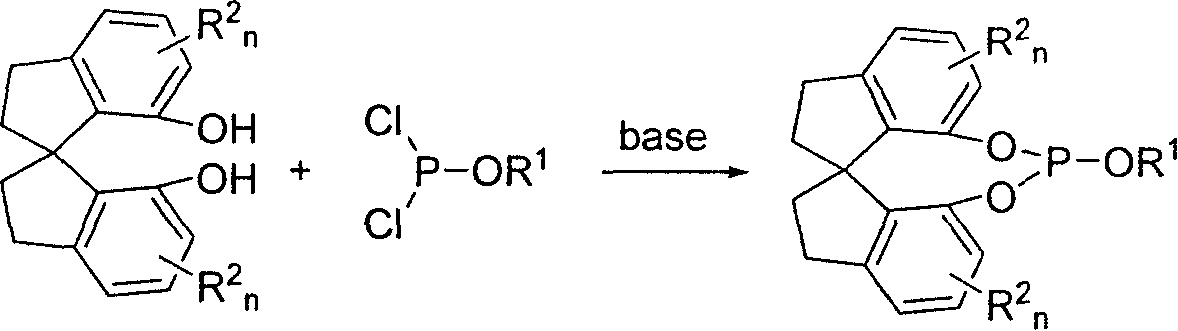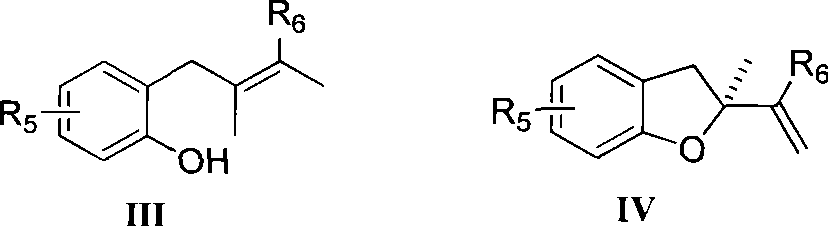Patents
Literature
139 results about "Axial chirality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
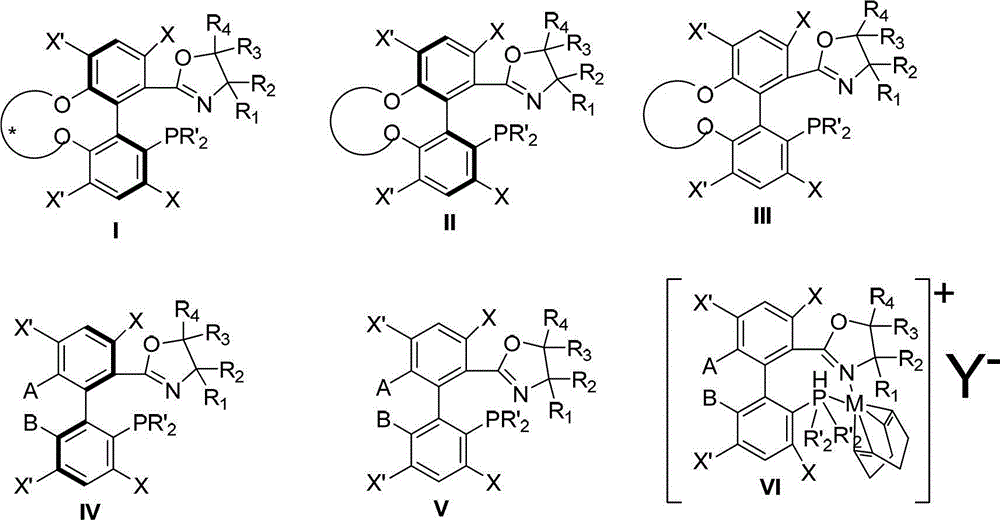
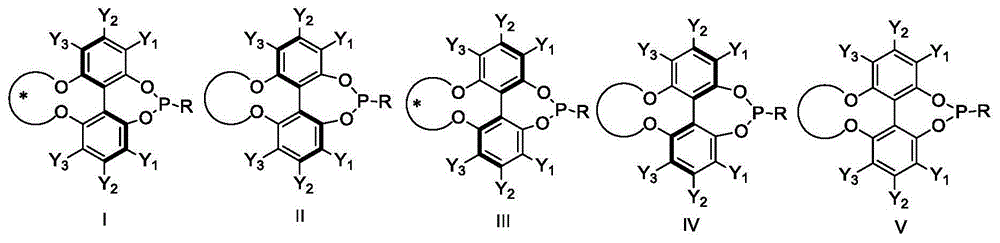
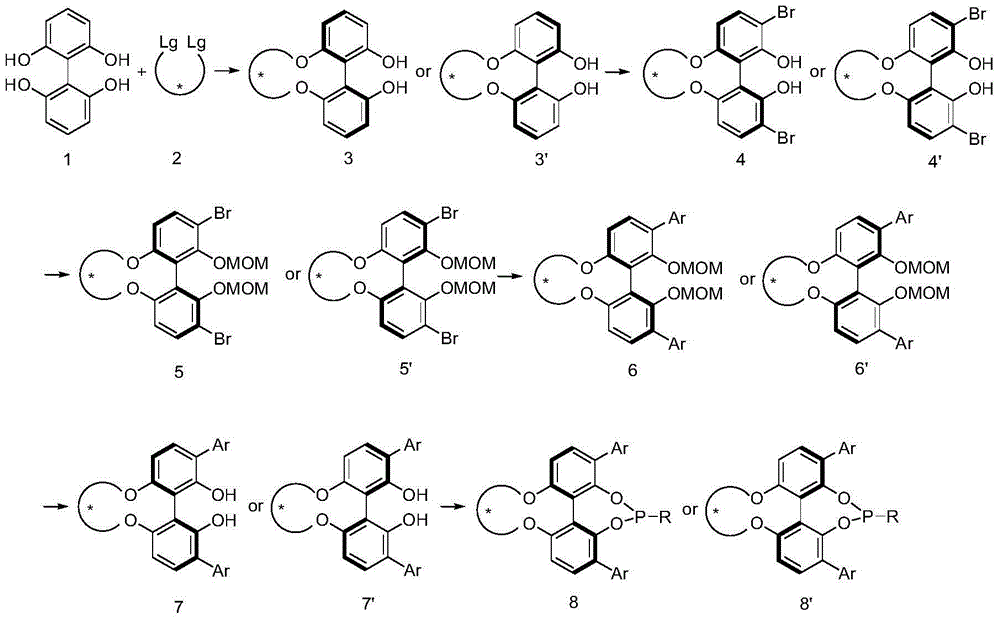
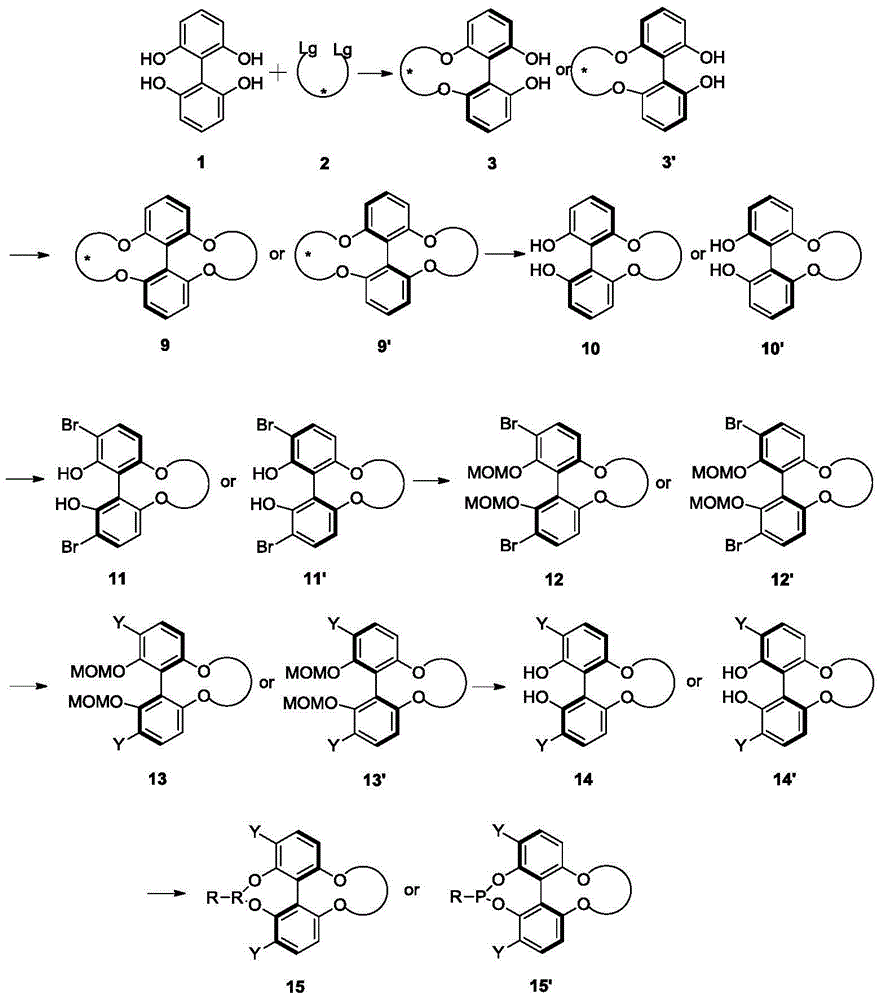
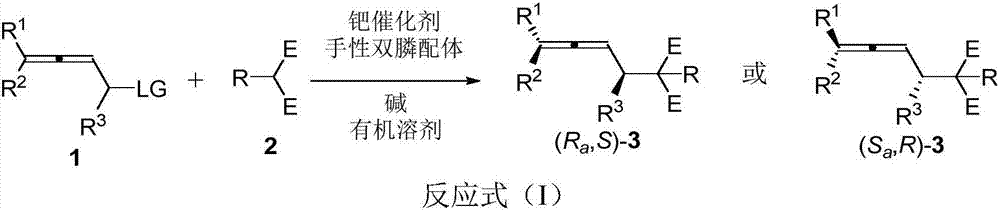
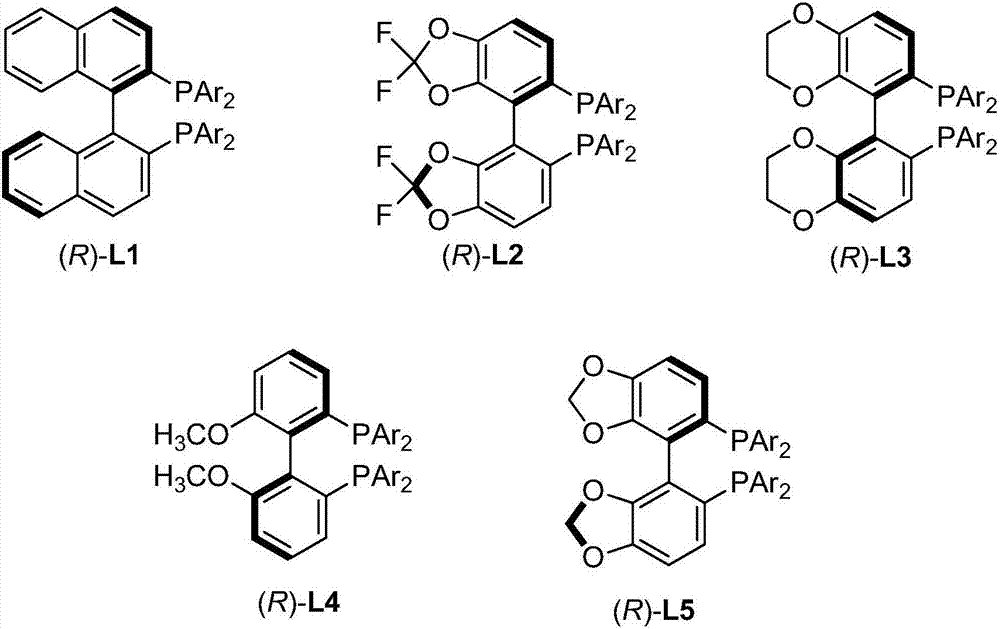
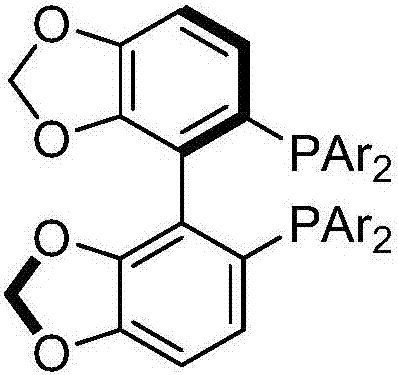
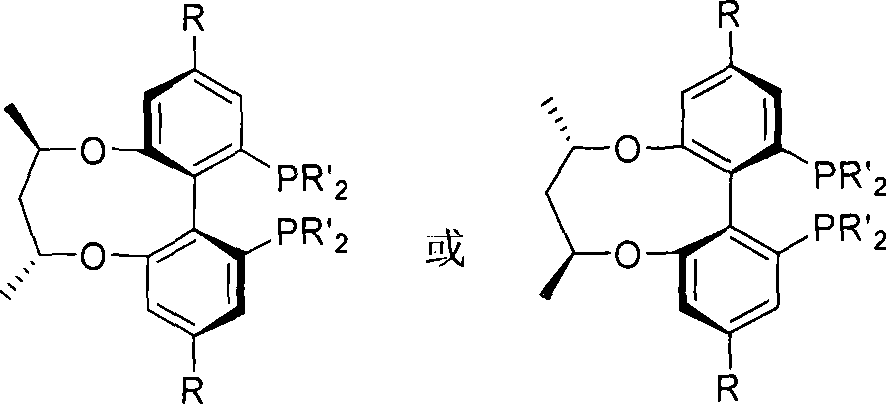
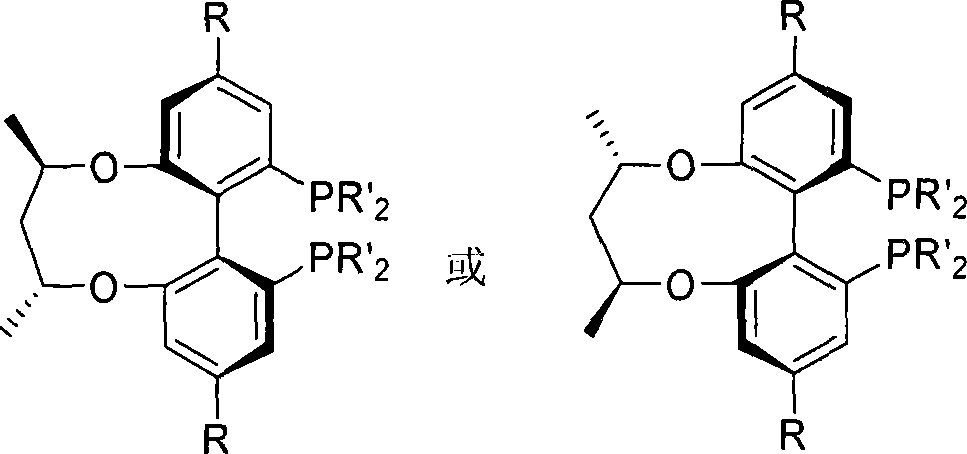
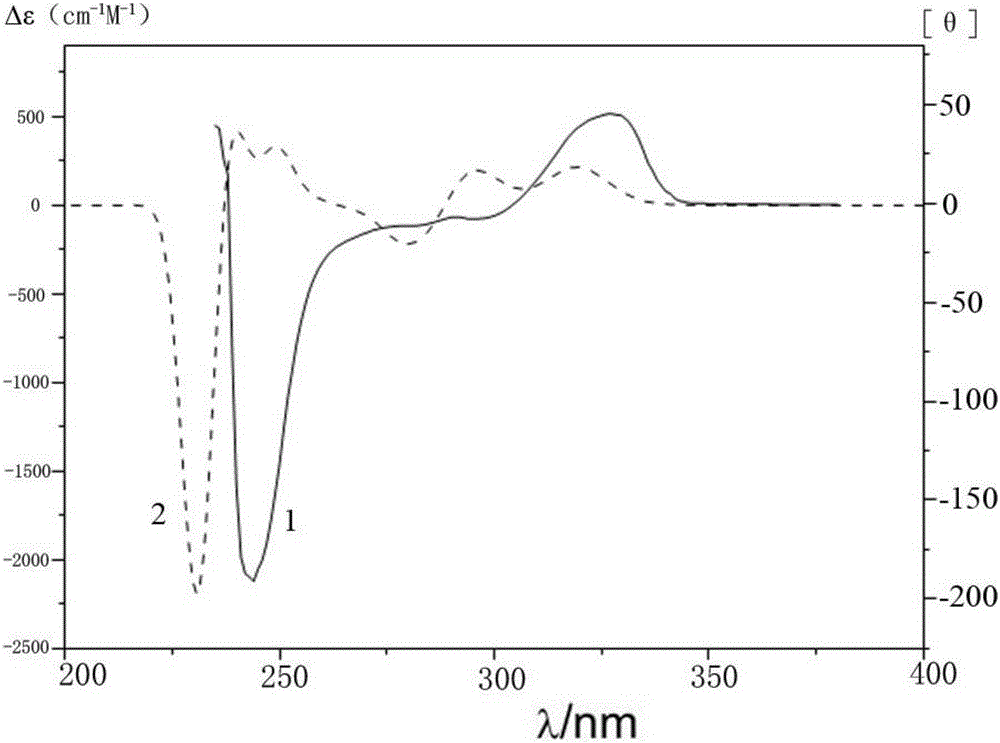
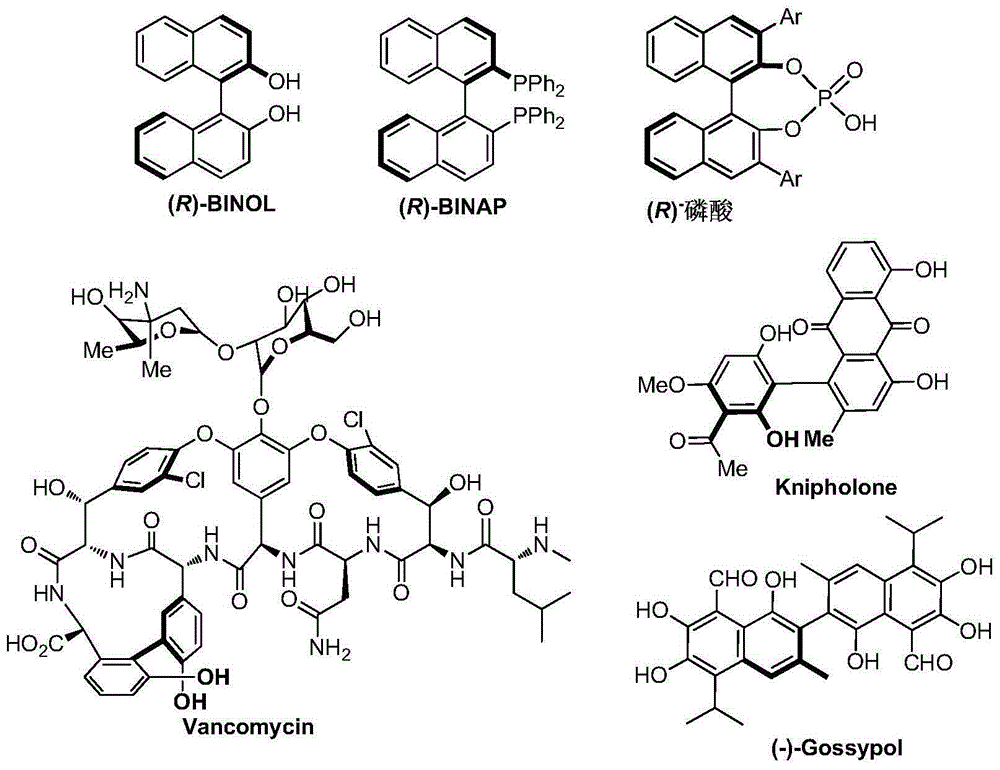
Axial chirality is a special case of chirality in which a molecule does not possess a stereogenic center (the most common form of chirality in organic compounds) but an axis of chirality, an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image. Axial chirality is most commonly observed in atropisomeric substituted biaryl compounds wherein the rotation about the aryl–aryl bond is restricted, for example, various biphenyls, binaphthyls such as BINAP, and certain dihydroanthracenone compounds. Certain allene compounds and spirans also display axial chirality. The enantiomers of axially chiral compounds are usually given the stereochemical labels Rₐ and Sₐ. The designations are based on the same Cahn–Ingold–Prelog priority rules used for tetrahedral stereocenters. The chiral axis is viewed end-on and the two "near" and two "far" substituents on the axial unit are ranked, but with the additional rule that the two near substituents have higher priority than the far ones.
Axial chiral isomers and preparation method and pharmaceutical application thereof
InactiveCN105622531ALow costOrganic active ingredientsSkeletal disorderMedicinal chemistryCombinatorial chemistry
Owner:MEDSHINE DISCOVERY INC
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
Phosphine ligand and enantiomer or racemic body thereof and preparation methods thereof
ActiveCN102532196AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention discloses a phosphine ligand and an enantiomer or a racemic body thereof and preparation methods and applications thereof. The structural formulae of the phosphine ligand and the enantiomer or the racemic body are shown in the specifications; a phosphine ligand compound has a novel framework; complete transfer of planar chirality to axial chirality in a synthesis process is realized through a desymmetrization reaction; a synthesis method is simple and economical; during preparation of a chiral ligand, common complex chiral splitting processes are avoided; and an obtained chiral ligand has the advantages of high reaction activity, high enantioselectivity and the like in a model reaction, can be applied to catalytic reactions of a plurality of metals such as palladium, rhodium, nickel, copper, iridium, ruthenium, iron, cobalt, gold, platinum and the like, and can have a very good catalytic effect.
Owner:SUN YAT SEN UNIV
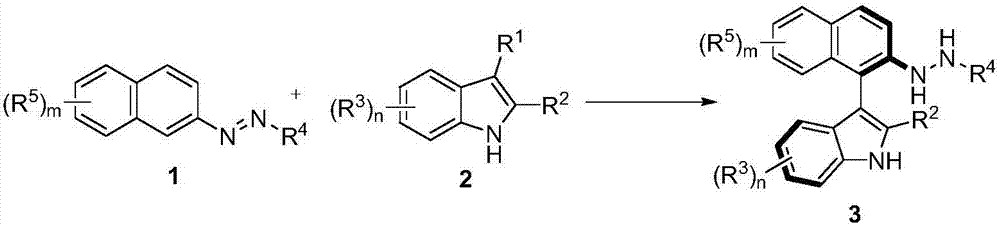
Method for synthetizing axial chirality aryl indole through organocatalysis
The invention discloses a method for synthetizing axial chirality aryl indole through organocatalysis. The method is characterized in that chiral phosphoric acid is used as a catalyst; a compound 1 and a compound 2 react: the chemical formula is shown as the description, wherein R<1> is hydrogen; R<2> is selected from tertiary butyl, 1-methyl cyclopropyl, 1-methyl cyclobutyl, tertiary pentyl, aryl and heteroaryl; R<3> represents any substituent group; n represents an integer being 1 to 4, and when n is greater than 2, existent two or greater R<3> are identical or different; R<4> selects CO2R, and R is alkyl or benzyl; R<5> represents any substituent group; m represents an integer being 1 to 4, and when m is greater than 2, existent two or greater R<5> are identical or different. The synthesis method is applicable to azobenzene derivatives of various kinds of ester; the axial chirality aryl indole is obtained at good yield and excellent enantioselectivity; the reaction conditions are mild. The method opens up a novel path for organocatalysis of asymmetrical aromatic functionalization.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
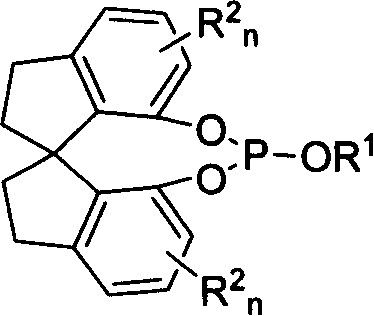
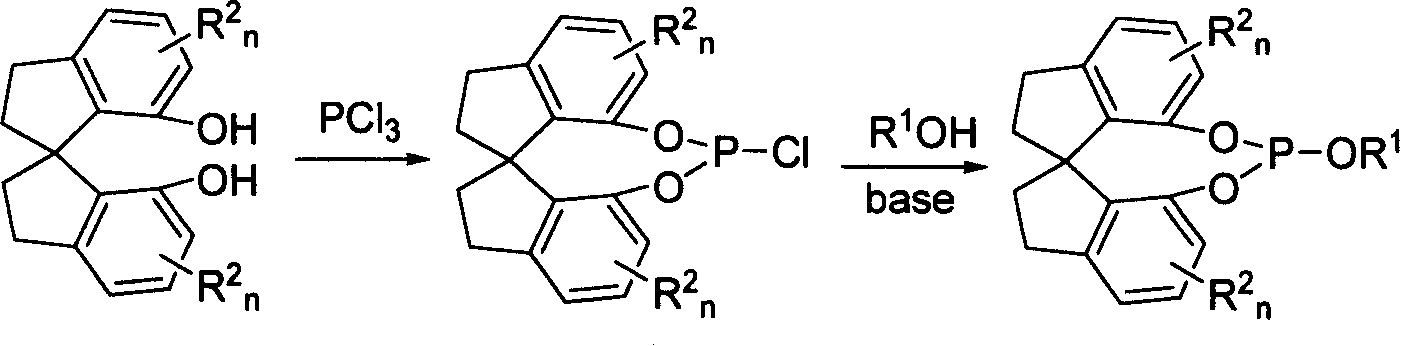
New type spirocyclic phosphic ester, preparation method and application in asymmetric addition reaction
A spirocyclosulphite is prepared from spirocyclodiphenol through three methods. Its spirodihydroindene structure has axial chirality, so said compound has two mutamers: levo- and dextro- spirocyclosulphite compounds. It can be used for the asymmetrical addition reaction of aldehyde and imine with high stereo activity.
Owner:NANKAI UNIV
3, 3'-position biaryl group binaphthyl shaft chiral phosphoramidite ligand and preparation method thereof
InactiveCN101565436AGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventPhosphorus trichloride
The invention relates to a 3, 3'-position binaphthyl shaft chiral phosphoramidite ligand containing biaryl group and a preparation method thereof. The preparation method comprises the following steps of: leading 3, 3'-dual-biaryl group-2, 2'-dinaphthol to react with hexagon-alkyl group phosphoramidite in organic solvent at the temperature of 0-120 DEG C according to the mol rate of 1:1, or leading 3, 3'- dual-biaryl group-2, 2'-dinaphthol to react with phosphorus trichloride and dialkyl amine in steps, and after complete reaction, the object chiral phosphoramidite ligand can be obtained through washing, extraction and separation. After going through complexation with metallic copper salt, the chiral phosphoramidite ligand can be used for catalyzing the conjugate addition reaction of zinc alkyl with alpha, beta-unsaturated carbonyl compounds, and an additional product is prepared with high yield up to 78-98% and high enantioselectivity ee value up to 80-98%.
Owner:TIANJIN UNIV
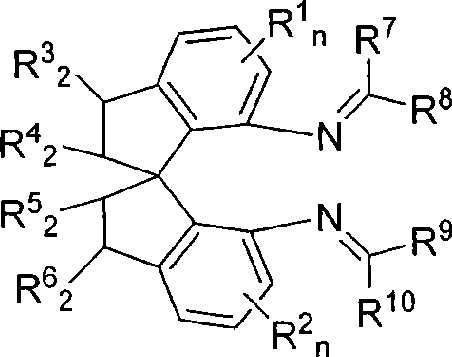
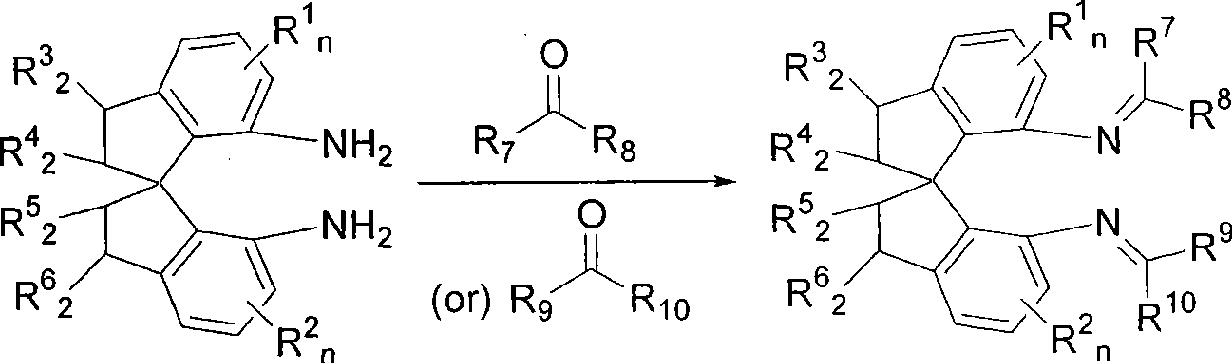
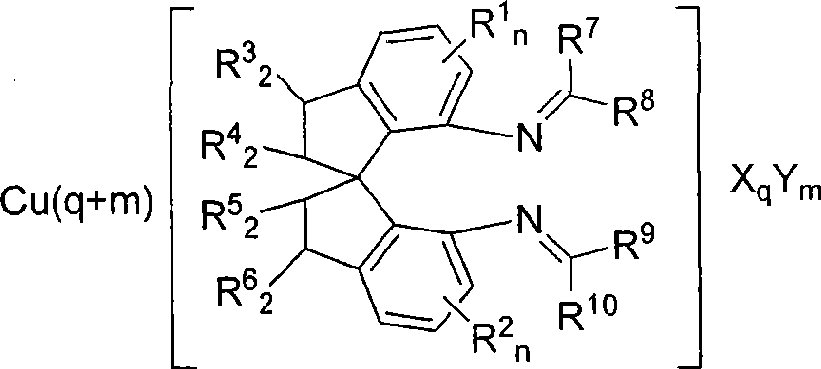
Spiro bisimine and preparation method and application thereof
InactiveCN101519356ASilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCarbeneInsertion reaction
The invention relates to a spiro bisimine and a preparation method and application thereof. Spiro diamine is adopted as raw material to generate spiro bisimine. The spiro skeleton structure in the spiro bisimine has axial chirality, therefore, the class of compound has two optical isomers, namely, right spiro bisimine and left spiro bisimine; and the commensurable mixture of the two optical isomers become racemization spiro bisimine. The complex compound of the spiro bisimine and copper can be taken as a chiral catalyst which is used in asymmetric insertion reaction of carbene to silicon-hydrogen bond, and has very high activity and enantio selectivity (as high as 99.2 percent ee). The chemical formula of the spiro bisimine is as follows: n=0-3; and the values of R<1>, R<2>, R<3>, R<4>, R<5>, R<6>, R<7>, R<8>, R<9> and R<10> are defined as claim of right 1.
Owner:EAST CHINA UNIV OF SCI & TECH
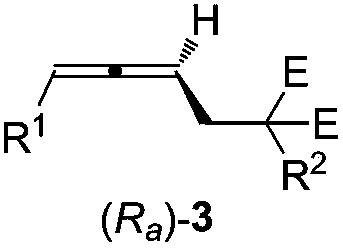
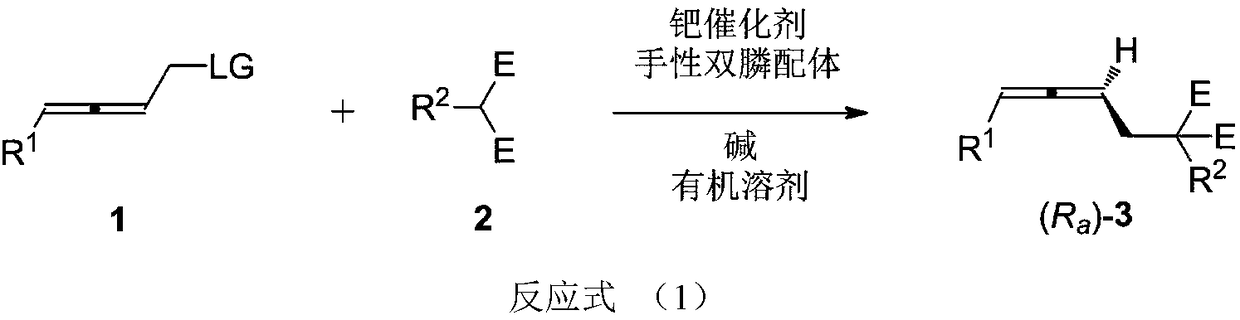
High-optical-activity axial chirality allene compound and construction method thereof
ActiveCN108976123AWide applicabilityImprove compatibilityOrganic compound preparationOrganic chemistry methodsPalladium catalystDiphosphines
The invention relates to a high-optical-activity axial chirality allene compound and a construction method thereof. The construction method is a method for directly constructing an allene compound with axial chirality under the high stereoselectivity by performing a reaction of 2,3-allene functional group compound and a nucleophilic reagent in an organic solvent under the action of a palladium catalyst, a chiral diphosphine ligand and alkali. The method has the advantages of simplicity for operation, easiness for obtaining raw materials and a reagent and the like; the substrate universality iswide; high stereoselectivity of the product is very excellent (90 to 96%ee). The high-optical-activity allene product obtained by the method disclosed by the invention is used as an important intermediate and can be used for constructing chiral compounds, such as gamma-allenoate, gamma-allenoic acid, gamma-allenol and gamma-butyrolactone, and gamma-butyrolactone natural product (R)-traumatic lactone (98%ee) is synthesized under high enantioselectivity for the first time.
Owner:ZHEJIANG UNIV
Alpha-menaphthyl substituted spiro bis(oxazoline) ligands, synthetic method and application thereof in synthesizing pyrazolidine derivatives
ActiveCN101560191AThe synthesis method is simpleMild conditionsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic cyclisationRegioselectivityChirality
The invention provides spiro bis(oxazoline) ligands with a plurality of chiral centers, a preparation method and application. The ligands are provided with axial chirality of a spiro backbone and central chirality of an oxazoline ring. The ligands can be prepared by condensation of chiral spiro diacid and corresponding alkamine. The invention also provides application of the spiro bis(oxazoline) ligands in synthesizing pyrazolidine derivatives in high regioselectivity and high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Phosphoramidite ligand as well as preparation method and application thereof
ActiveCN104610363AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumCatalytic effect
The invention relates to the field of chemical catalysis and discloses a phosphoramidite ligand as well as a preparation method and an application thereof. The synthesized phosphoramidite ligand and an enantiomer or a racemate of the phosphoramidite ligand take biphenyl as a framework and are subjected to an asymmetric induced reaction of axial chirality to realize complete transfer from central chirality to axial chirality. A synthetic method for the phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is simple and economic, and a common and complex chiral resolution process is avoided during chiral ligand preparation. The phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is employed to form the ligand together with active metal-iridium for catalyzing an asymmetric addition reaction of N-protected isatin and arylboronic acid, a very good catalytic effect is achieved, and a product can obtain the yield of more than 96% and the enantiomeric excess (ee) value of more than 92%.
Owner:SUN YAT SEN UNIV
High optical activity allene compound combing axial chirality and central chirality, construction method and application thereof
InactiveCN107488089AHigh selectivityWide applicabilityGroup 4/14 element organic compoundsOrganic compound preparationPalladium catalystDiphosphines
The invention discloses a method for directly constructing a high optical activity allene compound combing axial chirality and central chirality. Under effects of a palladium catalyst, a chiral diphosphine ligand and alkali, 2,3-allene function group compounds shown in a formula (1) and a nucleophilic reagent in a formula (2) are reacted in an organic solvent, and the high optical activity allene compound combing axial chirality and central chirality can be directly constructed by one step. The method has the advantages of simple operation, easy acquisition of raw material and the reagent, mild reaction condition, wide substrate universality, and good function group compatibility, and the stereoselective performance of the product is excellent (greater than 99:1 d.r., 95-greater than 99% ee). The high optical activity allene compound can be taken as an important intermediate for constructing the compounds such as monofluoro methylation allene, gamma-allene acid ester, gamma-allene acid, gamma-allenol, and gamma-butanolide containing several chiral centers.
Owner:ZHEJIANG UNIV
Central chirality induced axial chirality diphosphine ligand and method for synthesizing same
InactiveCN101230075AAvoid splittingEasy to synthesizeGroup 5/15 element organic compoundsDiphosphinesStructural formula
The invention relates to central chiral induced axial chiral diphosphine ligand, which adopts the structural formula that in the formula, R1 is benzyl or substituted benzyl; R is tertiary butyl or trifluoromethyl. The invention adopts the preparation method that 1-R-3, 5-dibromo benzene is used as the raw material, to produce 3-R-5- bromophenol; and then methylation is performed to obtain 1-R-3- bromine -5- anisole; after lithium realization of 3-dihydro pjosphinyl-5-R methyl phenoxide which reacts with dihydro phosphonic chloride and is obtained through oxidation, the product of the reaction with iodine reacts with boron tribromide, 2-iodine-3-dihydro pjosphinyl-5-R phenol obtained through demethylation and then reacts with central chiral 2, 4- pentanediol derivative, to obtain 2, 4-di (2-iodine-3- dihydro pjosphinyl-5- substituent phenoxy oxygen) pentane; <6, 61 -(2, 4- pentanediol oxygen)>-(4, 41)- disubstituent -(2, 21)-di (dialkyl pjosphinyl)-(1, 11)- biphenyl is produced through coupling, and finally reaction is performed with tri-butylamine and trichlorosilane, to obtain the diphosphine ligand.
Owner:WUHAN UNIV
Chirality phosphine nitrogen compound containing N-aryl as well as synthetic method and application thereof
ActiveCN103087106AEfficient synthesisImprove efficiencyGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumPhosphorus trichloride
The invention provides a chirality phosphine nitrogen compound containing N-aryl as well as a synthetic method and application thereof. The phosphine nitrogen compound is based on axial chirality or comprises a helical chirality skeleton, and is synthetized by taking optically pure axial chirality or helical chirality glycols compound, phosphorus trichloride and aromatic secondary amine as raw material for reaction. The raw material used in the method is easy to obtain, the reaction condition is mild, and the products are easy to separate and purify. The N-aryl phosphine nitrogen compound base on the axial chirality or the helical chirality skeleton synthetized by the method can be effectively applied in substitution reaction of allyl catalyzed by metal iridium, so that the product can be obtained with high enantioselectivity and regioselectivity, and the substrate scope of the product is greatly widened.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
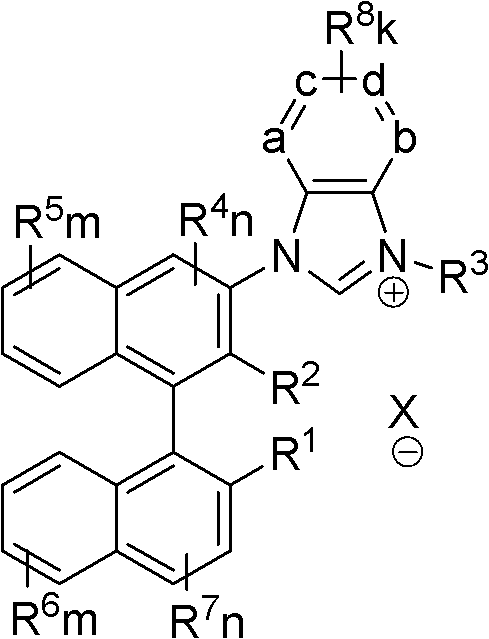
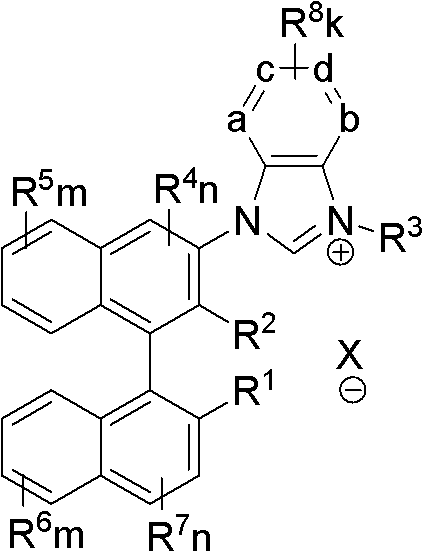
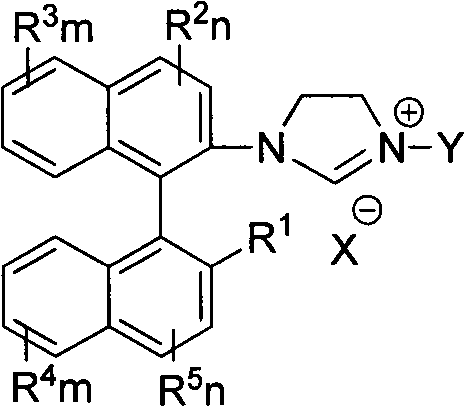
Axial chiral imidazole salt compound and preparation method thereof
InactiveCN102491947AProduct yield is highRaw materials are cheap and easy to getGroup 5/15 element organic compoundsArylTrifluoroacetic acid
The invention provides an axial chiral imidazole salt compound and a preparation method thereof. The structural formula of the axial chiral imidazole salt compound is that a binaphthol derivative and halogen reacts firstly to obtain a intermediate product which is coupled with o-phenylenediamine to obtain a single substituted axial chiral o-phenylenediamine compound and a axial chiral o-phenylenediamine compound with symmetrical C2. The single substituted axial chiral o-phenylenediamine compound and a heterocyclic aromatic halogenated compound or an aryl trifluoroacetic acid ester compound react to obtain an intermediate product, and the intermediate product and ortho-acid trialkyl ester react to obtain the axial chiral imidazole salt compound with R3 which is aryl. The single substituted axial chiral o-phenylenediamine compound and the ortho-acid trialkyl ester react to obtain a intermediate product, the intermediate product and a halogenated alkyl compound react to obtain the axial chiral imidazole salt compound with R3 which is alkyl. The axial chiral imidazole salt compound preparation method is high in synthesis yield, raw materials are cheap and easy to obtain, and the chiral center of the obtained axial chiral imidazole salt compound is close to a reaction point, thereby being favorable for obtaining high asymmetrical selective results.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing chiral dihydrobenzofuran compounds and catalyst used thereby
InactiveCN101412702AOvercome limitationsAvoid issues such as wasteOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesChemical industryBenzene
The invention relates to a method for preparing a chiral dihydrobenzofuran compound and a catalyst used by the same, which belongs to the technical field of chemical industry. The method prepares a chiral dihydrobenzofuran derivative IV by using a 2-allyl phenols compound III as a raw material and using an axial chiral metal complex as a catalyst in a reaction solvent in the presence of an oxidant, wherein the structural formula of the compounds III and IV are as above. The method uses the metal complex as the catalyst which has higher reactivity and enantioselectivity up to 99 percent and can be applied to various asymmetric catalytic reactions, wherein R5 is hydrogen, phenyl, naphthyl, halogen, benzyl, or alkyl with carbons of between 1 and 8; and R6 is hydrogen, phenyl, naphthyl, benzyl, or alkyl with carbons of between 1 and 8.
Owner:SHANGHAI JIAO TONG UNIV +1
Organic small molecule catalyst of aqueous phase asymmetric direct aldol condensation reaction, and preparing method and use thereof
InactiveCN101011671AHigh yieldHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCyclohexanoneDiastereomer
The invention discloses an organic molecular catalyst of water-phase asymmetrical hydroxy-aldehyde condensing reaction, which is characterized by the following: possessing central chirality and axial chirality to catalyze asymmetrical hydroxy-aldehyde condensing reaction in the water phase; improving receiving rate; obtaining the product with diastereomer selectivity and high-optical purity.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
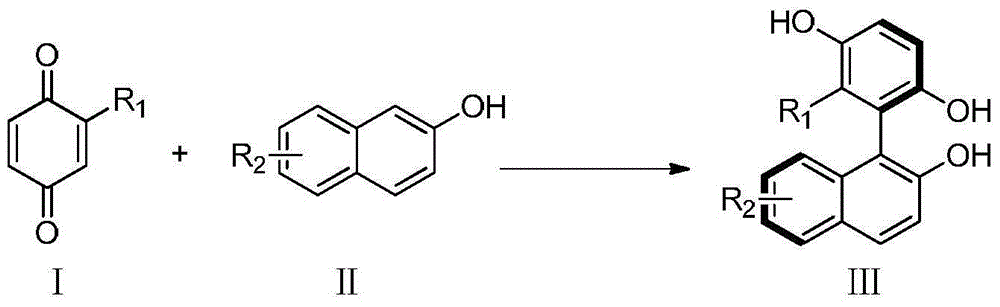
Method for catalyzing asymmetrically synthesized axially chiral biaryl diphenol
ActiveCN105152934AEasy to useEasy to manufactureOrganic compound preparationCarboxylic acid esters preparationPhosphoric acid2-Naphthol
The invention discloses a method for catalyzing asymmetrically synthesized axially chiral biaryl diphenol. In an organic solvent, chiral phosphoric acid is taken as a catalyst, a compound shown in a formula III is obtained by reaction on a compound shown in a formula I and a compound shown in a formula II, wherein R1 is alkoxyl, an ester group, halogen, alkyl, hydroxyl, aryl, a formyl group or amide; R2 is alkoxyl, cyano, an ester group, alkyl, aryl, halogen or H. According to the method disclosed by the invention, in a mild reaction condition, simple and easily prepared raw materials are used to synthesize the axially chiral biaryl diphenol with a high yield. The synthetic method disclosed by the invention has good enantioselectivity and the ee value of the obtained product is very high; the synthetic method disclosed by the invention can endure various functional groups and a substituent of 2-naphthol can be an electron-withdrawing substituent or an electron donating substituent. The catalyst used in the method is convenient in source and free of heavy metal pollution, and has an industrialized prospect. The formulae are shown in the description.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
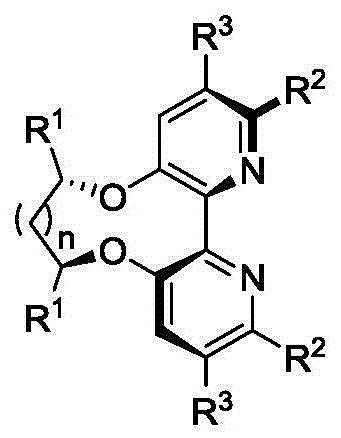
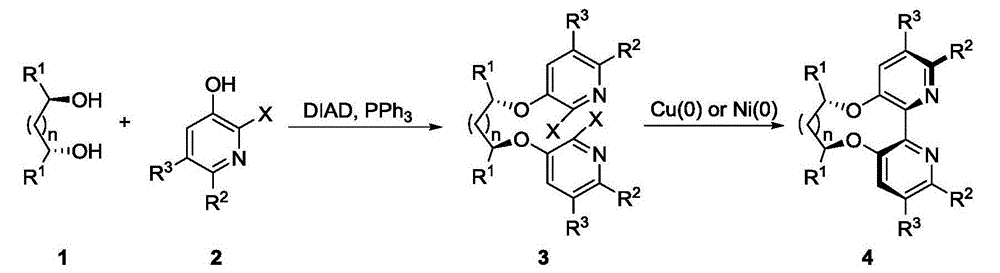
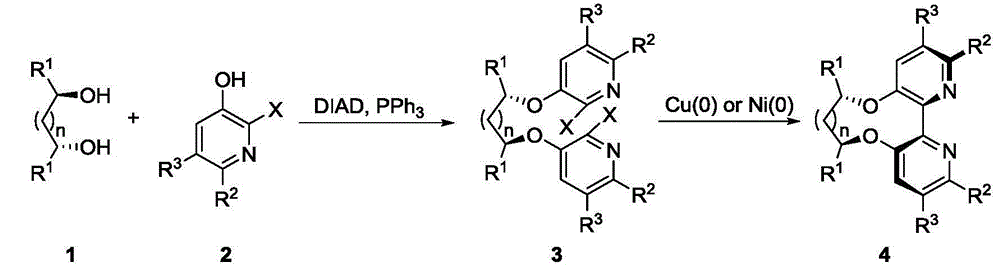
Dipyridine ligands with axial chirality and synthetic method thereof
The invention relates to dipyridine ligands with axial chirality. A synthetic method adopts 3-hydroxy-2-halogen pyridine as an initial raw material, and includes loading a chiral skeleton with the pyridine through a Mitsunobu reaction with a chiral diol, and achieving pyridine coupling through an Ullman reaction catalyzed by nickel (0) or copper (0) to obtain the dipyridine ligands induced by the chiral diol. The method is simple and convenient in operation and high in yield. The synthetic method is more practical when being compared with traditional methods.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Axial chirality diphosphine ligand containing bis-schiff base
InactiveCN101392006AHigh reactivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsDiphosphinesStructural formula
The invention relates to an axis-chiral double phosphine ligand containing double schiff bases in the chemical technical field; the structural formula is shown as follows. The axis-chiral double phosphine ligand comprises the axis-chiral of biphenyl, can be used in all metal accelerating asymmetric reactions such as asymmetric epoxidation reaction of olefin, asymmetric cyclopropanation and the like, and has higher reactivity (can reach over 70 percent ) and stereoselectivity (can reach over 80 percent).
Owner:SHANGHAI JIAO TONG UNIV
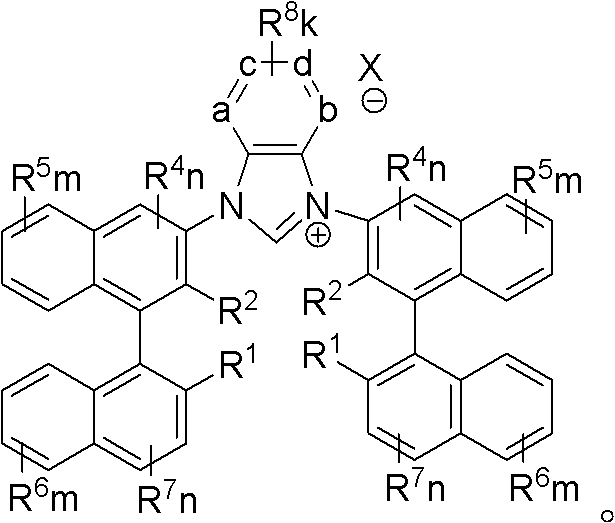
Axially chiral imidazole salt compound and preparation method thereof
InactiveCN102070529ANovel structureImprove stabilityGroup 5/15 element organic compoundsEvaporationSolvent
The invention provides an axially chiral imidazole salt compound and a preparation method thereof. The axially chiral imidazole salt compound has a structural formula shown in the specifications. The technical scheme of the preparation method comprises the following steps of: dissolving a binaphthyl axially chiral imidazole diamine compound in an alcohol acid mixed solvent for acidification, and removing the solvent by evaporation so as to obtain a solid intermediate product; drying the solid intermediate product in a drier which contains P2O5, and dissolving in raw acid trialkyl ester for reaction under the protection of inert gas; and after the reaction is finished, cooling reaction liquid to room temperature, precipitating a solid product, filtering the solid product, and washing with Et2O. The alcohol is absolute methanol or absolute ethanol; and the acid is any one of haloid acid, HBF4, HPF6 or HOTf. The axially chiral imidazole salt compound can be applied to asymmetric oxidation reactions, asymmetric coupling reactions and asymmetric addition reactions.
Owner:SOUTH CHINA UNIV OF TECH
Axial chirality bis-schiff base-containing ligand
InactiveCN101391970AHigh reactivityHigh stereoselectivityImino compound preparationAsymmetric synthesesStructural formulaCyclopropanation
The invention relates to an axis chiral ligand containing bis-Schiff bases in the field of chemical technology, the structural formula thereof is as follows, in the formula, R1 equals to hydrogen, chlorine, methyl, ethyl, isopropyl, tertiary butyl or alkoxyl with1 to 3 carbons; R2 equals to hydrogen, chlorine, methyl, ethyl, isopropyl, tertiary butyl or alkoxyl with1 to 3 carbons. The invention has the axis chirality of biphenyl compounds, can be applied to asymmetric reactions catalyzed by metals, such as asymmetric cyclopropanation and the like, and has higher reactivity ( can achieve more than 90 percent ) and stereoselectivity ( can achieve more than 85 percent ).
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing axial chirality diaromatic compound with optical activity
InactiveCN101531578AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNatural productCoupling reaction
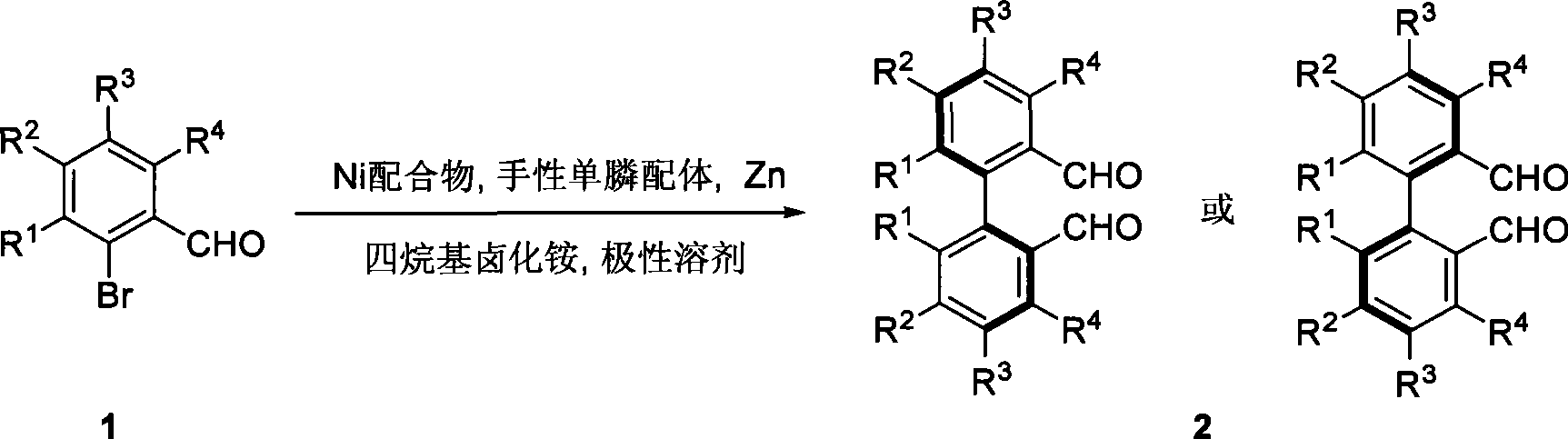
The invention relates to a method for preparing an axial chirality diaromatic compound with optical activity, which is realized by an asymmetric Ullmann-type coupling reaction catalyzed by chiral monophosphine ligand and nickel composition. The method has the advantages of mild reacting condition, simple and convenient operation and good zymolyte applicability, can prepare the axial chirality diaromatic compound in high stereoselectivity and high yield, and has application prospect of mass synthesis of natural products with axial chirality.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Chiral binaphthalene-aza-polycyclic ligand and preparation method thereof
InactiveCN112142638AAxichiralCentral chiralityOrganic compound preparationOrganic chemistry methodsPtru catalystCarboxylic acid
The invention discloses a chiral binaphthalene aza-polycyclic ligand and a preparation method thereof. The ligand has the following structure. The preparation method comprises the following steps: (1)by taking 1, 1 '-binaphthalene 2, 2'-diphenol as a raw material, carrying out methyl protection, Tf2O protection, methylation, aromatic ring substitution and bromination reaction to obtain a 2-bromomethyl -2'-methoxy 1, 1'-binaphthalene derivative (compound V); (2) starting from aza-polycyclic carboxylic acids such as L -or D-proline, L -or D-2-piperidinecarboxylic acid, L- or D-acridine -acid and the like, carrying out derivatization to obtain various amino alcohols (compounds B); and (3) coupling the compound V and the compound B under an alkaline condition and a catalyst, and removing a protecting group to obtain the binaphthalene aza-polycyclic chiral ligand L. The ligand has central chirality and axial chirality at the same time, and can obtain higher reaction activity and enantioselectivity in asymmetric catalytic reaction. The ligands are simple to prepare, raw materials are easy to obtain, and the ligands have important significance for asymmetric synthesis.
Owner:NANKAI UNIV
Synthesis method of 1,3-disubstituted allene with high optical activity
ActiveCN104193568AReduce dosageWide applicabilityCarbamic acid derivatives preparationCarboxylic acid esters preparationSynthesis methodsAlkyne
The invention discloses a synthesis method of 1,3-disubstituted allene with high optical activity, namely a method for preparing 1,3-disubstituted allene with high optical activity in one step of functionalizing terminal alkyne, aldehyde and chiral alpha, alpha-diphenyl-L-prolinol under catalysis of a bivalent copper salt. The method is simple to operate, adopts easily available raw materials and reagents, uses a substrate with wide universality, can be compatible with a plurality of functional groups, such as a plurality of glycosidic units, primary alcohol, secondary alcohol, tertiary alcohol, amides and dimethyl malonate, and does not need further protection; and the obtained axial-chirality allene is moderate to good in yield and excellent in diastereoselectivity or enantioselectivity.
Owner:ZHEJIANG UNIV
Axial chirality-based binaphthol-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene complex and preparation method thereof
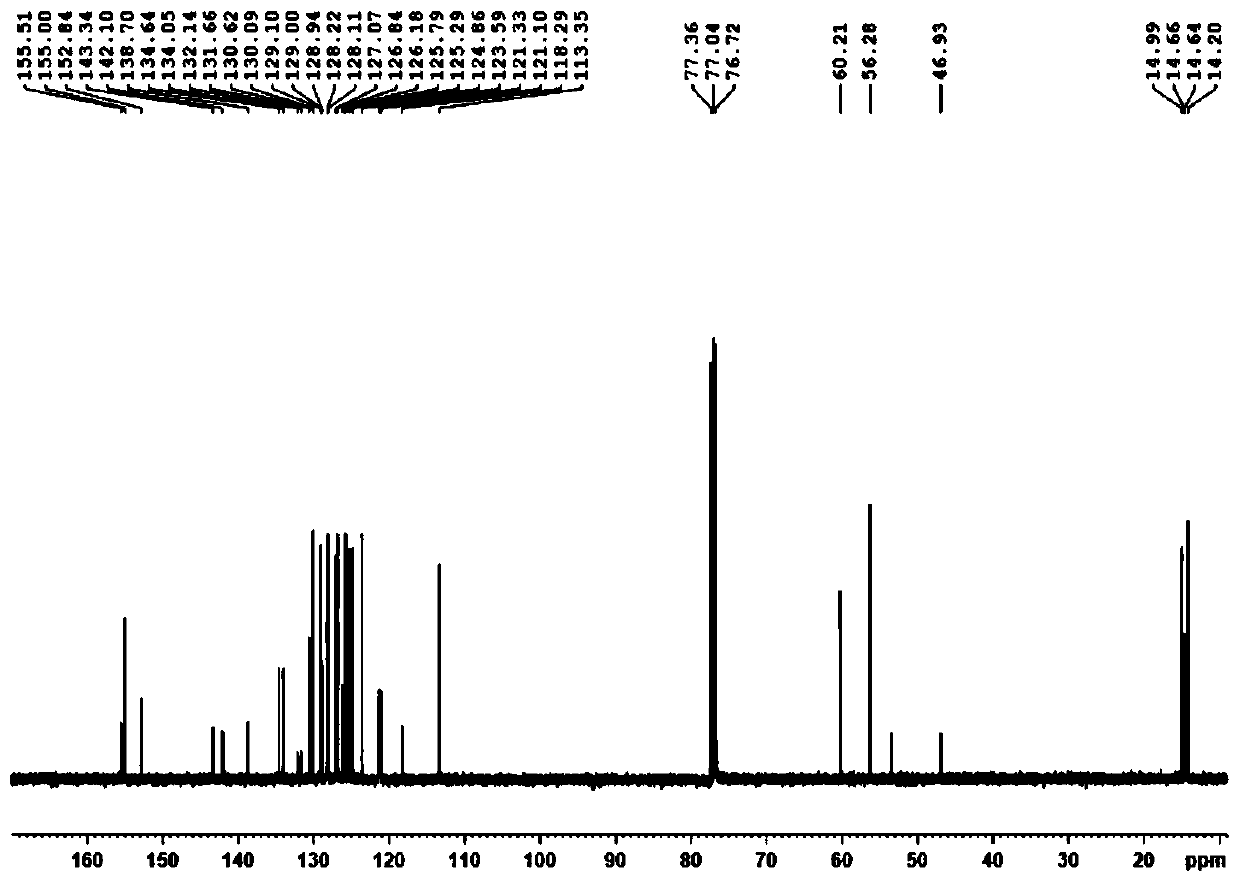
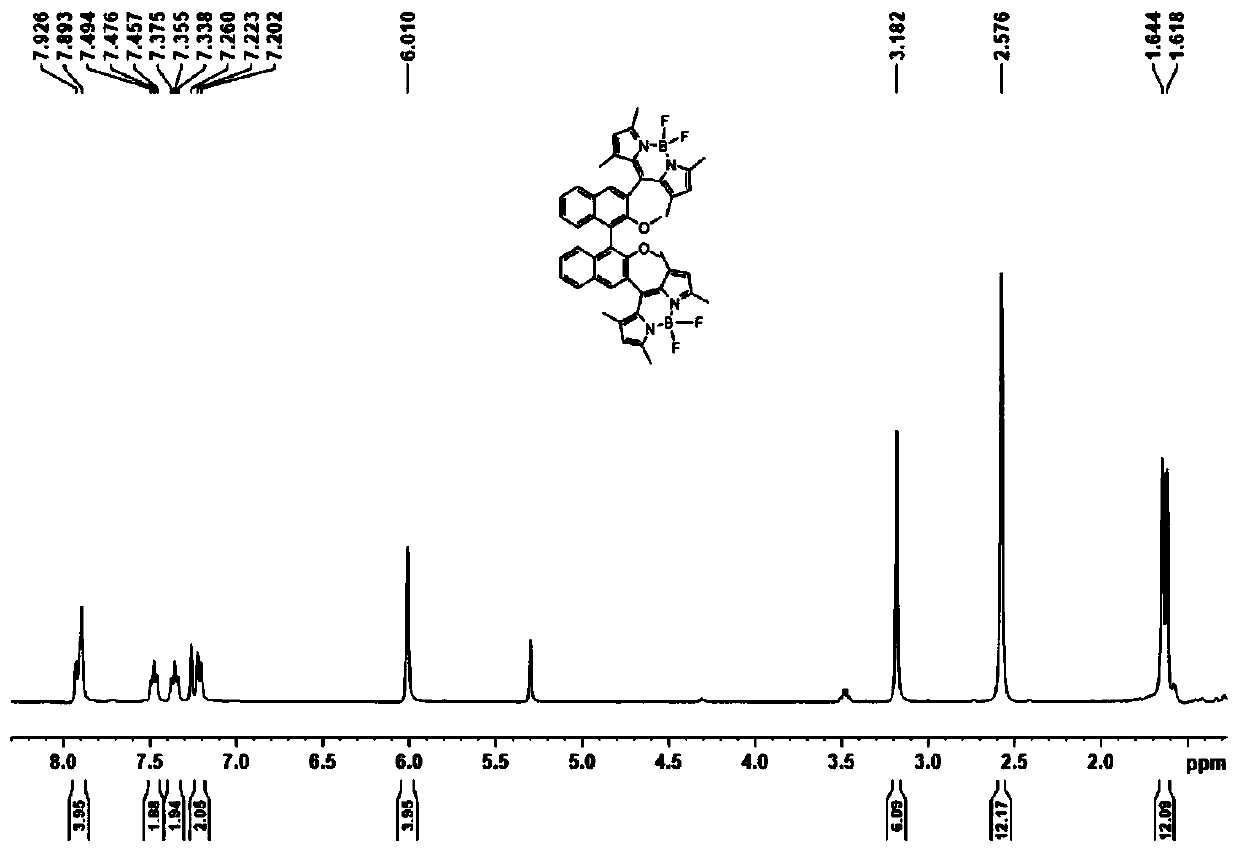
ActiveCN110669065AReduce manufacturing costMild reaction conditionsGroup 3/13 element organic compoundsLuminescent compositionsPolymer scienceDiethyl ether
The invention discloses an axial chirality-based binaphthol-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene complex and a preparation method thereof. The preparation method comprises the following steps: adding binaphthol into a first solvent, adding sodium carbonate and iodomethane, and performing a reaction so as to obtain 2,2'-dimethoxy-1,1'-binaphthalene; adding the 2,2'-dimethoxy-1,1'-binaphthalene into a second solvent, adding n-butyllithium and N,N-dimethyl formamide at -78 DEG C to 0 DEG C, increasing the temperature to room temperature, and performing a reaction so as to obtain 2,2'-dimethoxy-[1,1'-binaphthalene]-carboxaldehyde; adding the 2,2'-dimethoxy-[1,1'-binaphthalene]-carboxaldehyde into a third solvent, adding 2,4-dimethylpyrrole and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, and performing a stirring reaction; and adding triethylamine and a boron trifluoride diethyl etherate solution, and performing a reaction continuously for 5-10 hours, so as to obtain the desired complex. Through simple synthesis steps, the axial chirality-based binaphthol-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene complex is prepared, the preparation cost is low, the reaction condition is mild, purification is easy, and the synthesized compound has both an axial chirality structure of binaphthol and a fluorescence characteristic of a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene complex.
Owner:NANJING FORESTRY UNIV
Axial-chirality bidentate ligand and application thereof in copper-catalyzed coupling reaction
InactiveCN109336896AHigh activityLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDiethyl etherStructural formula
The invention discloses an axial-chirality bidentate ligand and application thereof in a copper-catalyzed coupling reaction. The structural formula of the axial-chirality bidentate ligand is shown inthe original specification, wherein R is 2-Me or 3-Ome or 32-t-Bu or 32-F or 32, 3, 4-F. The application of the axial-chirality bidentate ligand in the copper-catalyzed coupling reaction comprises thefollowing steps: stirring the ligand and copper salt in diethyl ether for coordination, concentrating and performing spin drying, so as to obtain a catalyst; adding trimethoxyphenyl-silicon and bromobenzene into a 10mL single aperture reaction flask, adding ethanol into the reaction flask for dissolving, adding alkali into the reaction flask and 5mol% of the catalyst, rising the temperature of the reaction to 60-100 DEG C, performing a reaction for 6-12h and directly performing column chromatography after the reaction is ended, so as to obtain a white solid product. According to the axial-chirality bidentate ligand and the application thereof in the copper-catalyzed coupling reaction, the technical problem that how the relatively high yield is obtained under a mild condition can be solved.
Owner:浙江工业大学上虞研究院有限公司
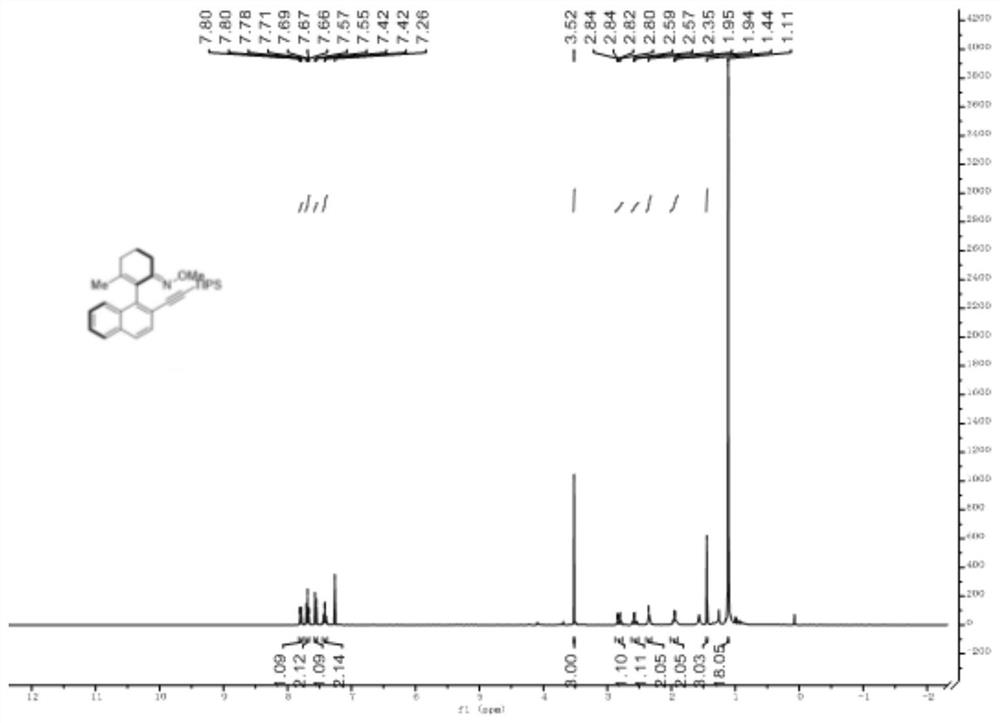
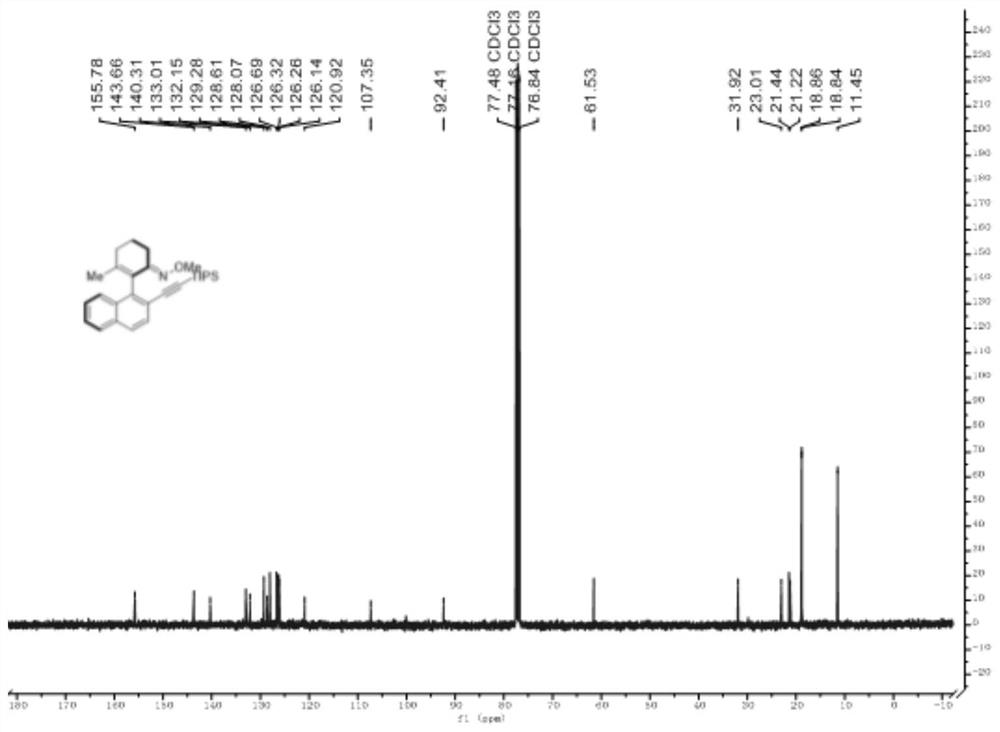
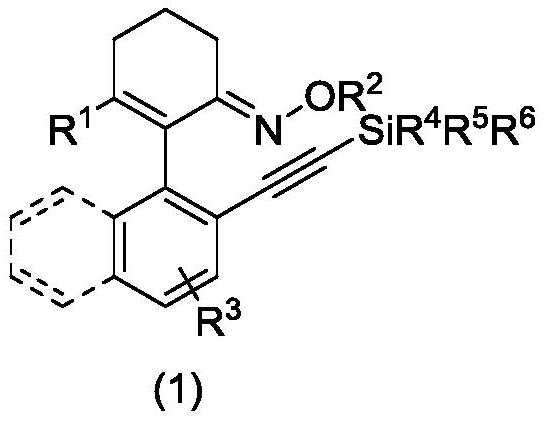
Axially chiral arylethynyl silane compound and preparation method thereof
ActiveCN112321627AReduce manufacturing costHigh yieldSilicon organic compoundsOrganic compound preparationPtru catalystOrganic synthesis
The invention relates to the technical field of organic synthesis, and discloses an axially chiral arylethynyl silane compound and a preparation method thereof, the structural formula of the axially chiral arylethynyl silane compound is shown in the formula (1), the preparation method is as follows: a complex formed by palladium salt and ligand is used as a catalyst precursor, by taking 2-aryl cyclohexene-1-ketoxime and 2-bromoethynyl silane as reactants, asymmetric alkynylation is carried out on a carbon-hydrogen bond in the reactants in a reaction medium in the presence of an oxidizing agentto obtain the axially chiral arylethynyl silane compound. The method has good adaptability to aryl and alkynyl silane containing substituent groups with different properties, a series of axial chiralaryl ethynyl silane compounds can be obtained with medium to good yield and enantioselectivity, and silicon substituent groups in the compounds are easy to convert in a series, and the derivatives can be used as chiral ligands and catalysts in asymmetric catalytic reactions.
Owner:HANGZHOU NORMAL UNIVERSITY
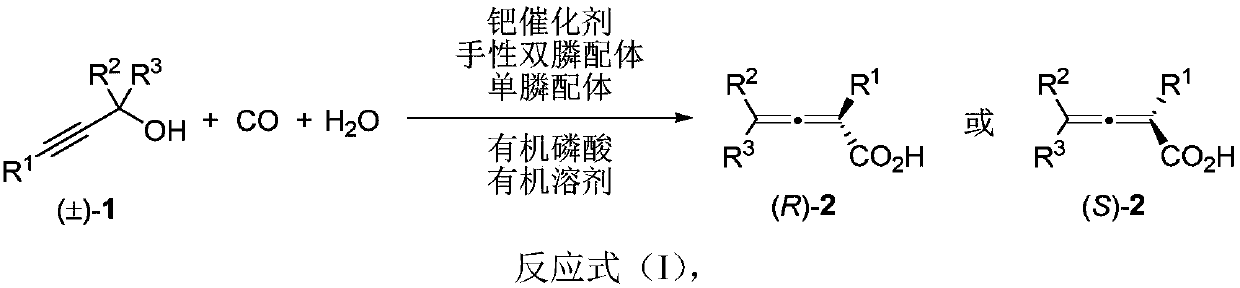
Method for directly constructing tetra-substituted allenic acid compound with high optical activity
ActiveCN111302928AHigh optical activityWide applicabilityCarboxylic acid nitrile preparationOrganic compound preparationO-Phosphoric AcidPtru catalyst
The invention discloses a method for directly constructing a tetra-substituted allenic acid compound with high optical activity, wherein tertiary propargyl alcohol, carbon monoxide and water react inan organic solvent under the actions of a palladium catalyst, a chiral diphosphine ligand, a monophosphine ligand and organic phosphoric acid to directly construct an axially chiral allenic acid compound with high optical activity in one step. The method has characteristics of simple operation, easily available raw materials and reagents, mild reaction conditions, wide substrate universality, goodfunctional group compatibility and high reaction enantioselectivity (90%-99% ee). According to the invention, the allenic acid compound with high optical activity can be used as an important intermediate for constructing compounds such as gamma-butyrolactone compounds containing tetra-substituted chiral quaternary carbon centers, tetra-substituted allenol and the like.
Owner:FUDAN UNIV
Chiral catalyst, process for preparing the same and its use in the oxidate coupling of naphthols
InactiveUS20050256345A1High optical purityOrganic compound preparationOrganic chemistry methodsCouplingBenzyl group
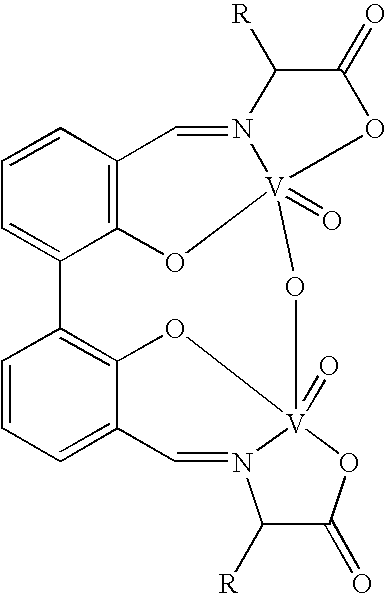
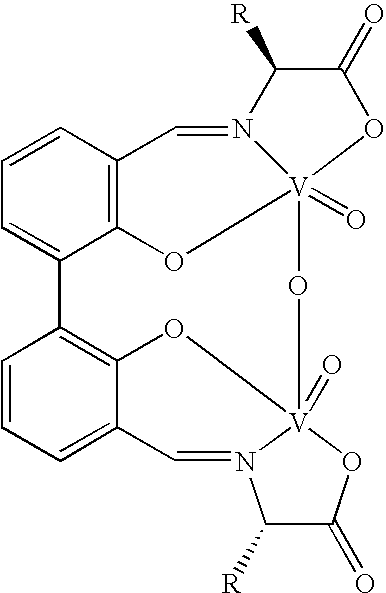
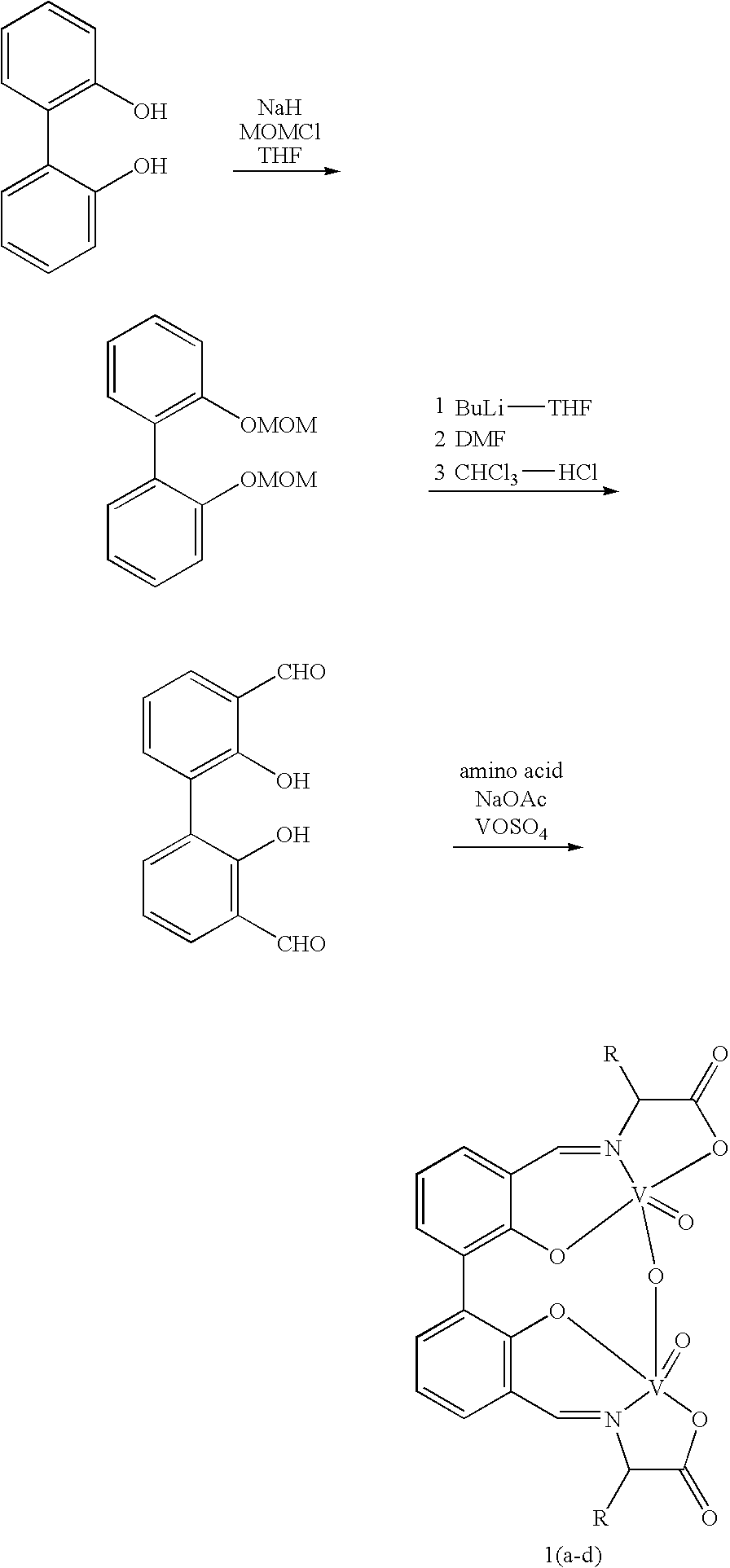
The compound of this invention is a useful catalyst for the oxidative coupling of naphthol. Its originality lies in that it is a novel vanadium complex of Schiff's base formed by a chiral amino acid and a formyl biphenol or its derivative. Its axis chirality is induced to form by the chiral amino acid. It has the general formula: where R represents a benzyl, an isopropyl, an isobutyl or a tertiary butyl and the configuration of the amino acid is R or S. The compound can catalyze oxidative coupling of naphthol or its derivative to form binaphthol or its derivatives with a high optical purity.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
1,1'-biphenyls axial chirality diphosphinidene amide ligand connected at 5,5' position
InactiveCN101168549AVariation range can be adjustedAsymmetric catalytic effect is goodGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenBromine
The invention relates to 1, 1(1)-diphenyl axial chiral bis di-phosphinous amide ligand connected at 5,5(1) position in the chemical technical field. The structural formula of the invention is shown as follows: in the formula, n is equal to 5, 6, 7, 8, 9, 10, 11, or 12; R1 is equal to hydrogen, fluorine, chlorine, bromine, iodine, trifluoromethyl, the alkyl of 1-8 carbon, or the alkoxyl of the 1-8 carbon; R2 is equal to the hydrogen, the fluorine, the chlorine, the bromine, the iodine, the trifluoromethyl, the alkyl of the 1-8 carbon, or the alkoxyl of the 1-8 carbon; R3 is equal to the hydrogen, the fluorine, the chlorine, the bromine, the iodine, the trifluoromethyl, the alkyl of the 1-8 carbon, or the alkoxyl of the 1-8 carbon; R4 is equal to the hydrogen, the fluorine, the chlorine, the bromine, the iodine, the trifluoromethyl, the alkyl of the 1-8 carbon, or the alkoxyl of the 1-8 carbon; R5 is equal to the hydrogen, the fluorine, the chlorine, the bromine, the iodine, the trifluoromethyl, the alkyl of the 1-8 carbon, or the alkoxyl of the 1-8 carbon; the ligand can be appliable to various unsymmetrical reactions catalyzed by metal, and have very high reaction activity and stereoselectivity.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com