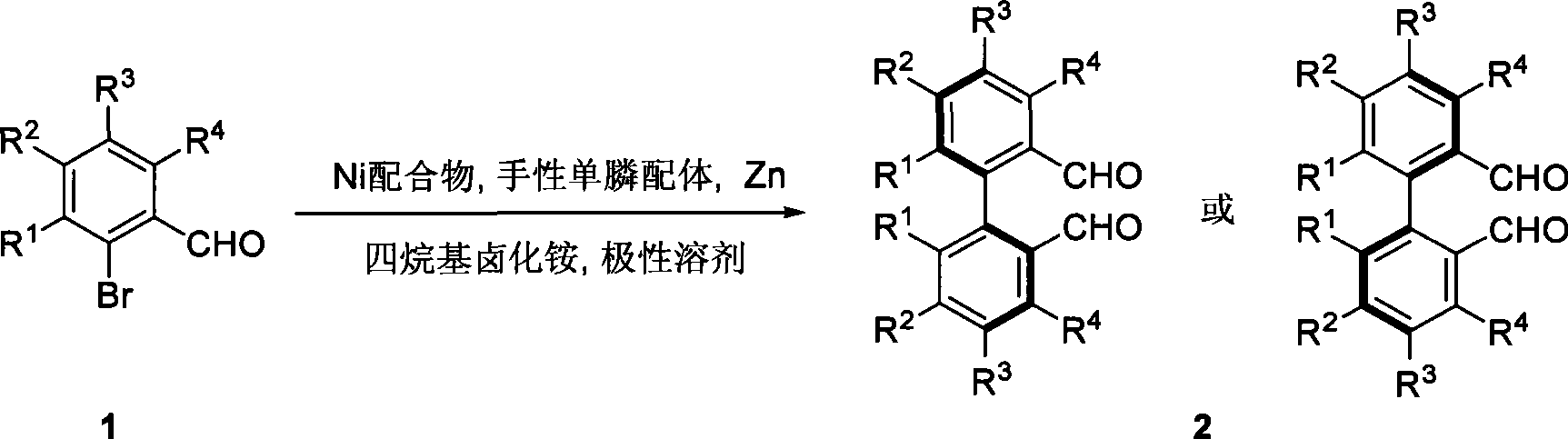
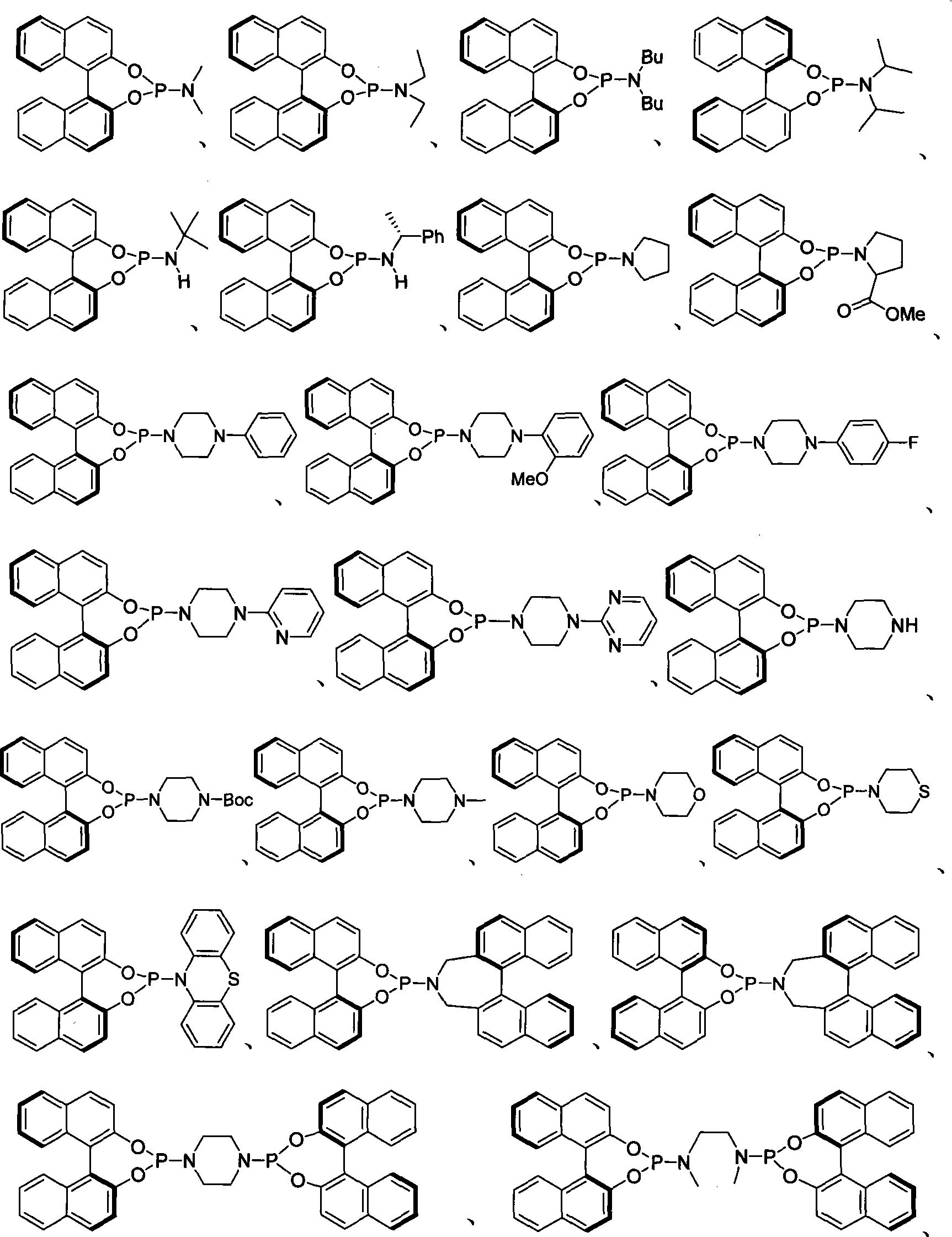
Method for preparing axial chirality diaromatic compound with optical activity
A compound and aromatic technology, which is applied in the field of preparation of optically active axial chiral aromatic compounds, can solve problems such as immature methods, inability to realize asymmetric self-coupling reaction of high-steric hindrance substrates, simple and practical reaction system, etc. , to achieve the effect of good applicability, simple reaction and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 2a
[0033] Pass high-purity argon into a dry 10-1000 milliliter (ml) reaction bottle, add NiCl 2 (PPh 3 ) 2 (0.02~20mmol), chiral monodentate phosphoramidite ligand (0.02~20mmol), activated zinc powder (0.4~400mmol), Bu 4 NI (0.1~100mmol), after pumping twice, add 0.5~500mL of anhydrous DMA, stir at room temperature for 10min, then add substrate 3,4,5-trimethoxy 2- Bromobenzaldehyde (0.2 ~ 200mmol), under the protection of argon, stirred at a certain temperature for a certain period of time, then cooled to room temperature, filtered with diatomaceous earth, and then filtered with CH 2 Cl 2 Wash the filter residue three times (3×15mL), wash the organic phase (3×20mL) with water, remove DMA as much as possible, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate, and purify by flash silica gel column chromatography to obtain the coupled product 2a. See Table 1 for rate and ee values (negative values indicate o...
Embodiment 2
[0039] Synthesis of 2a
[0040] Pass high-purity argon into the dry 10-1000ml reaction flask, add NiCl 2 (PPh 3 ) 2 (0.02~20mmol), chiral monodentate phosphoramidite ligand (S)-11 (0.02~20mmol), activated zinc powder (0.4~400mmol), Bu 4 NI (0.1~100mmol), after pumping twice, add 0.5~500mL of anhydrous DMA, stir at room temperature for 10min, then add substrate 3,4,5-trimethoxy 2- Bromobenzaldehyde (0.2 ~ 200mmol), under the protection of argon, stirred at a certain temperature for a certain period of time, then cooled to room temperature, filtered with diatomaceous earth, and then filtered with CH 2 Cl 2 Wash the filter residue three times (3×15mL), wash the organic phase with water (3×20mL), remove DMA as much as possible, wash the organic phase with saturated saline, dry over anhydrous sodium sulfate, concentrate, and purify by flash silica gel column chromatography to obtain the corresponding coupling product 2a, yield 67%, ee value 67%.
[0041]
[0042] (EA: calc...
Embodiment 3
[0044] Synthesis of 2b
[0045] The operation is the same as in Example 2, the yield is 55%, and the ee is 58%.
[0046] mL / min, detected at 254nm, t R1 = 11.4min(minor), t R2 =15.5min(major).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com