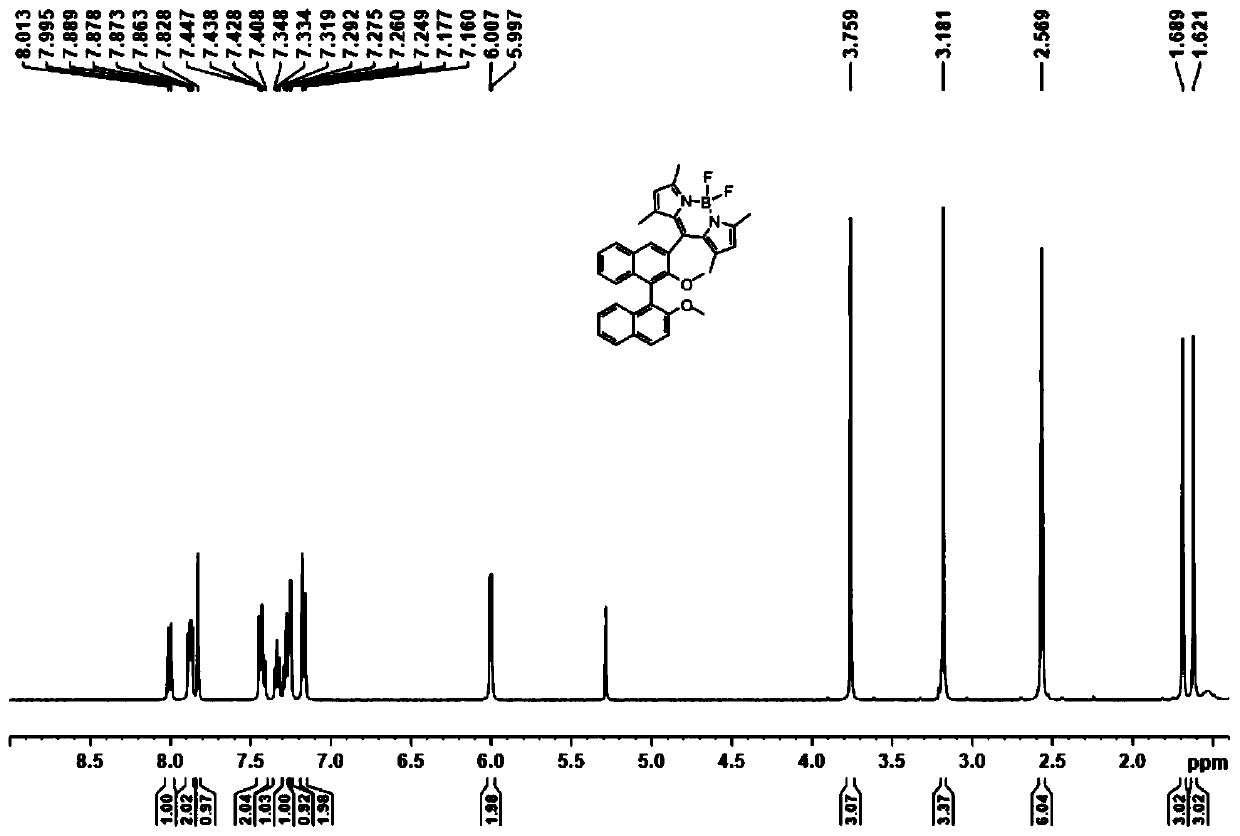
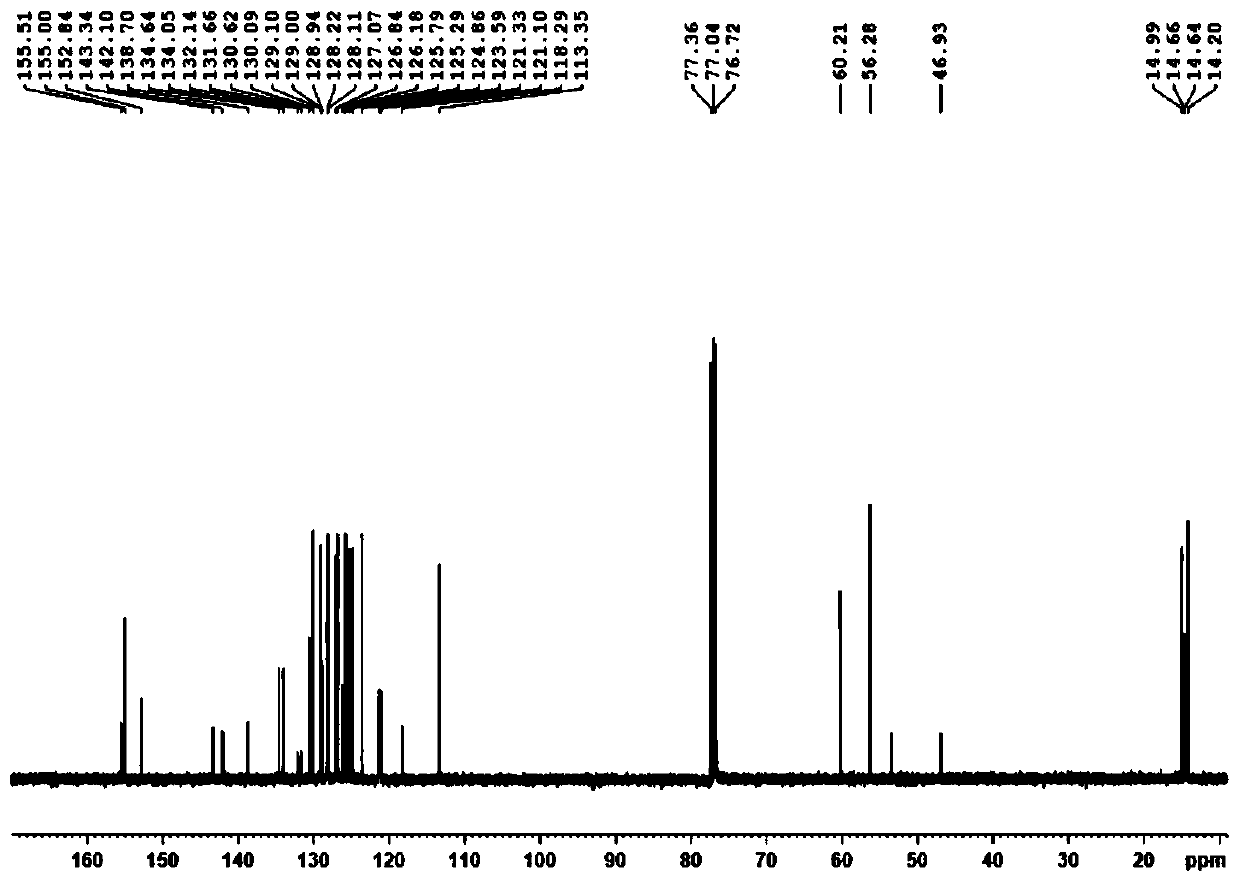
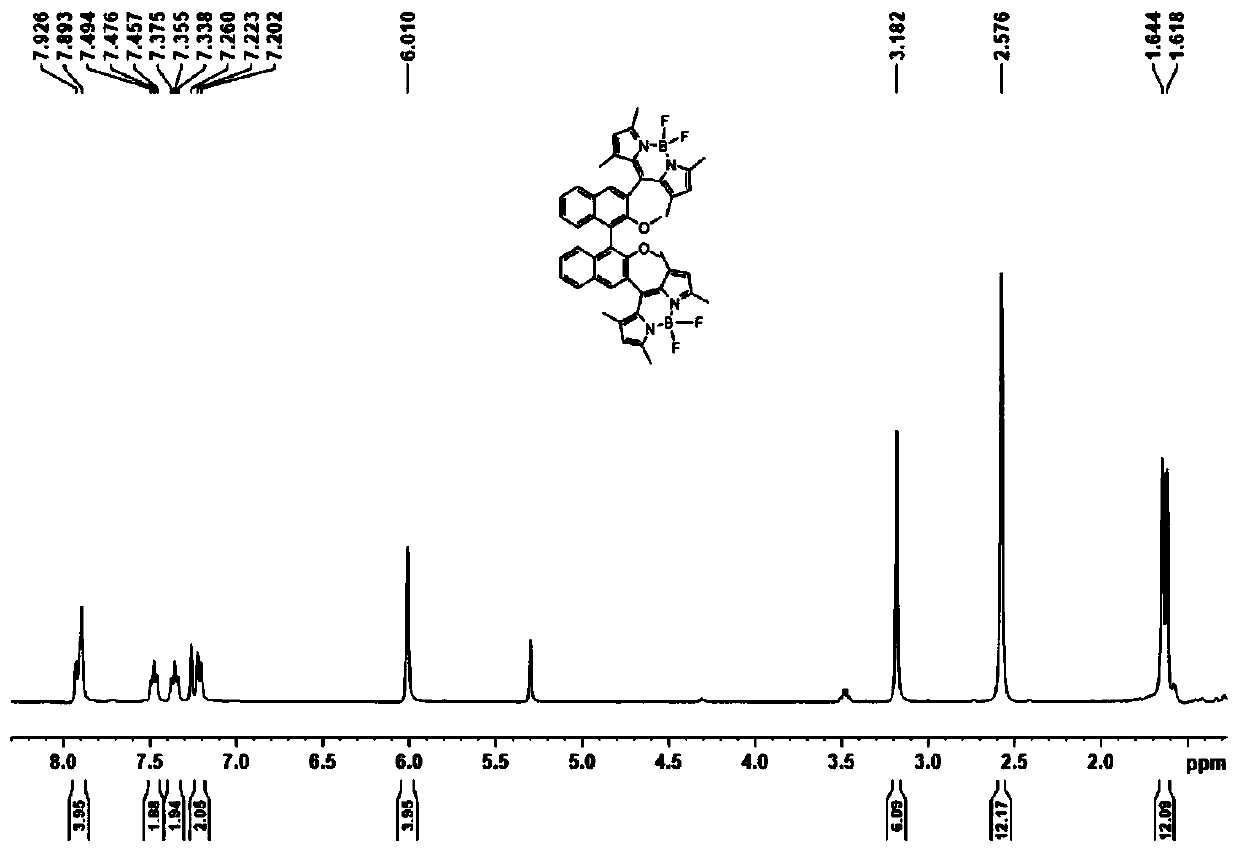
Axial chirality-based binaphthol-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene complex and preparation method thereof
A technology of fluoroborate dipyrrole and binaphthol, which is applied in the field of organic functional luminescent materials, can solve problems such as complex synthesis steps, luminescence quenching, and high cost, and achieve the effects of low preparation cost, mild reaction conditions, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A kind of preparation method based on axial chiral binaphthol-fluoroboron dipyrrole complex of the present invention comprises the following steps:
[0041] (1) Add binaphthol (BINOL) to the first solvent, add sodium carbonate and methyl iodide to react, and obtain 2,2'-dimethoxy-1,1'-binaphthyl;
[0042] (2) Add the 2,2'-dimethoxy-1,1'-binaphthyl to the second solvent, and add n-butyllithium and N,N-dimethyl Base formamide, after warming up to room temperature, react to obtain 2,2'-dimethoxy-[1,1'-binaphthyl]-aldehyde;
[0043] (3) Add the 2,2'-dimethoxy-[1,1'-binaphthyl]-aldehyde to the third solvent, add 2,4-dimethylpyrrole, 2,3-dichloro -5,6-dicyano-1,4-benzoquinone stirring reaction;
[0044] (4) Add triethylamine and boron trifluoride ether solution to step (3), and continue to react for 5-10 hours to obtain the desired complex.
[0045] Described reaction process is:
[0046]
Embodiment 1
[0048] Add single-configuration BINOL (14.32g, 50mmol), anhydrous acetone and sodium carbonate (23.3g, 168.6mmol) into the reactor, add iodomethane (12.2mL, 196mmol) after stirring for 10 minutes, and continue stirring at 60°C 24 hours. After the reaction was completed, the solvent was removed, suction filtered, washed with water, and dried to obtain 14.9g of 2,2'-dimethoxy-1,1'-binaphthalene.
[0049]Anhydrous and oxygen-free operation, add 2,2'-dimethoxy-1,1'-binaphthalene (2.36g, 7.5mmol) and 120mL anhydrous THF into the reactor and stir to dissolve, slowly add n-butyl at -78°C Lithium (1.5M, 6 mL, 9 mmol) was stirred for 1 hour, DMF (1.2 mL, 15.5 mmol) was added at this temperature, and the reaction was continued at room temperature for 5 hours. After the reaction, the solvent was spin-dried, extracted with ethyl acetate / water, and separated on a silica gel column with petroleum ether and ethyl acetate at a volume ratio of 4:1 to obtain 2,2'-dimethoxy-[1 ,1'-binaphthyl]-...
Embodiment 2
[0055] Add single-configuration BINOL (14.32g, 50mmol), anhydrous acetone and sodium carbonate (23.3g, 168.6mmol) into the reactor, add iodomethane (12.2mL, 196mmol) after stirring for 10 minutes, and continue stirring at 50°C 30 hours. After the reaction was completed, the solvent was removed, suction filtered, washed with water, and dried to obtain 14.2 g of 2,2'-dimethoxy-1,1'-binaphthalene.
[0056] Anhydrous and oxygen-free operation, add 2,2'-dimethoxy-1,1'-binaphthalene (1.3g, 4.1mmol) and anhydrous THF 90mL into the reactor and stir to dissolve, slowly add n-butyl at 0°C Lithium (2.5M, 7.3mL, 18.3mmol) was stirred for 3 hours, DMF (2.6mL, 33.1mmol) was added at this temperature, and the reaction was continued at room temperature for 6 hours. After the reaction, the solvent was spin-dried, extracted with ethyl acetate / water, and separated on a silica gel column with petroleum ether and ethyl acetate at a volume ratio of 3:1 to obtain 2,2'-dimethoxy-[1 ,1'-binaphthyl]-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com