Chiral binaphthalene-aza-polycyclic ligand and preparation method thereof
A technology of polycyclic rings and binaphthyls, which is applied in the field of chiral ligand preparation, can solve the problems of few applications in the field of asymmetric catalysis and uncommon binaphthyl ligands, and achieve the goal of fewer steps, improved catalytic performance, and enhanced coordination ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] Taking compound B2 as an example, its preparation method is introduced in detail, which specifically includes the following steps:
[0095] (1) Dissolve Boc-protected proline methyl ester in an organic solvent, add methylmagnesium bromide Grignard reagent under zero-degree conditions, and react at room temperature for 10 to 18 hours to generate Boc-protected aminoalcohol, and then Separation and purification by column chromatography; wherein the molar ratio of Boc-protected proline methyl ester and methylmagnesium bromide Grignard reagent is 1:4-6;
[0096] (2) Under zero-degree conditions, add 2N ethanol solution of hydrogen chloride to the product separated and purified in step (1) and stir at room temperature for 2 to 3 hours to obtain the corresponding amino hydrochloride, namely compound B2; wherein, Boc protected The molar ratio of amino alcohol and hydrogen chloride ethanol solution is 1:1.5~4.
[0097] Compound B3, Compound B4 and Compound B5 can be prepared re...
Embodiment 1
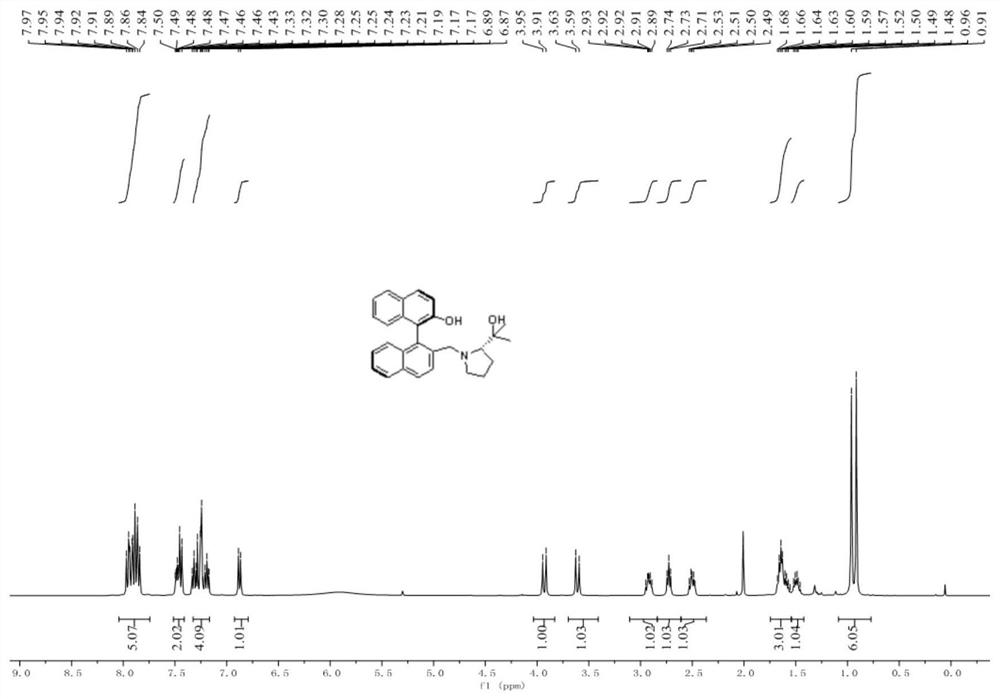
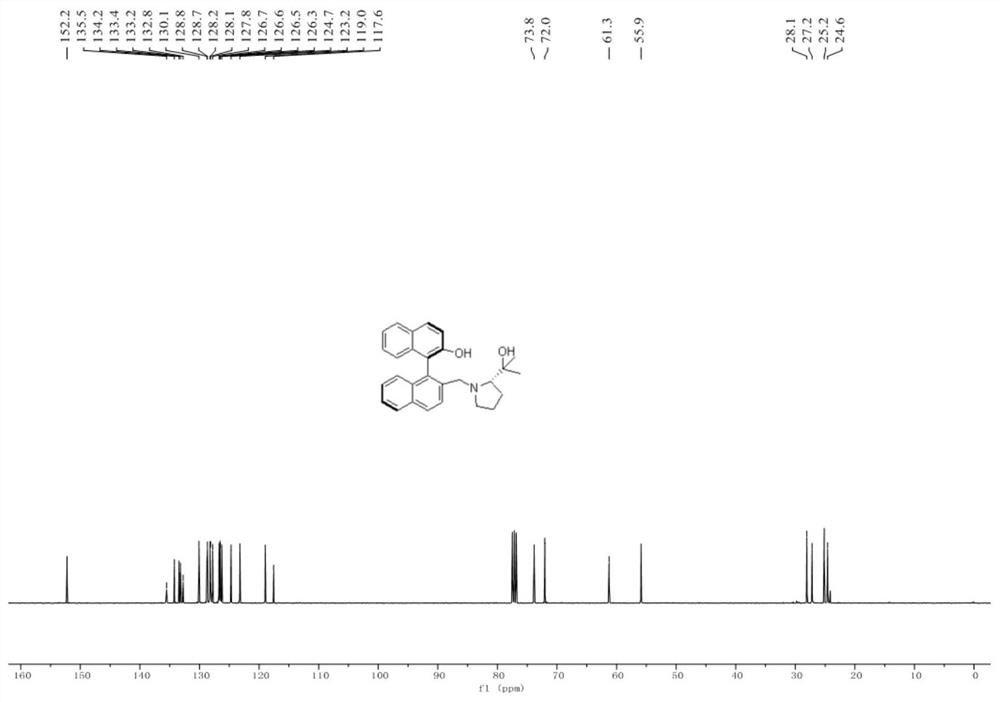
[0109] A novel chiral binaphthalene-aza five-membered ring ligand: (R a ,S)-2'-((2-(2-Hydroxypropan-2-yl)pyrrolidin-1-yl)methyl)-1,1'-binaphth-2-ol, number L-0, structural formula as follows:
[0110]
[0111] The preparation method of above-mentioned novel chiral binaphthaza five-membered ring ligand comprises the following steps:
[0112] 1. Preparation of compound IV-0
[0113] Synthesis of Compound I
[0114]
[0115]Dissolve (R)-1,1-binaphthol (1 equivalent) in acetone, add potassium carbonate (2 equivalents), slowly add iodomethane (1.05 equivalents) dropwise at room temperature, and heat to reflux for 12 h. After the reaction was detected by TLC, the reaction solvent was removed under reduced pressure, extracted 3 times with ethyl acetate and water, 1 time with saturated brine, dried over anhydrous magnesium sulfate, and oily liquid was obtained under reduced pressure, separated by column chromatography, petroleum ether: acetic acid Ethyl ester=10:1 washing, t...
Embodiment 2
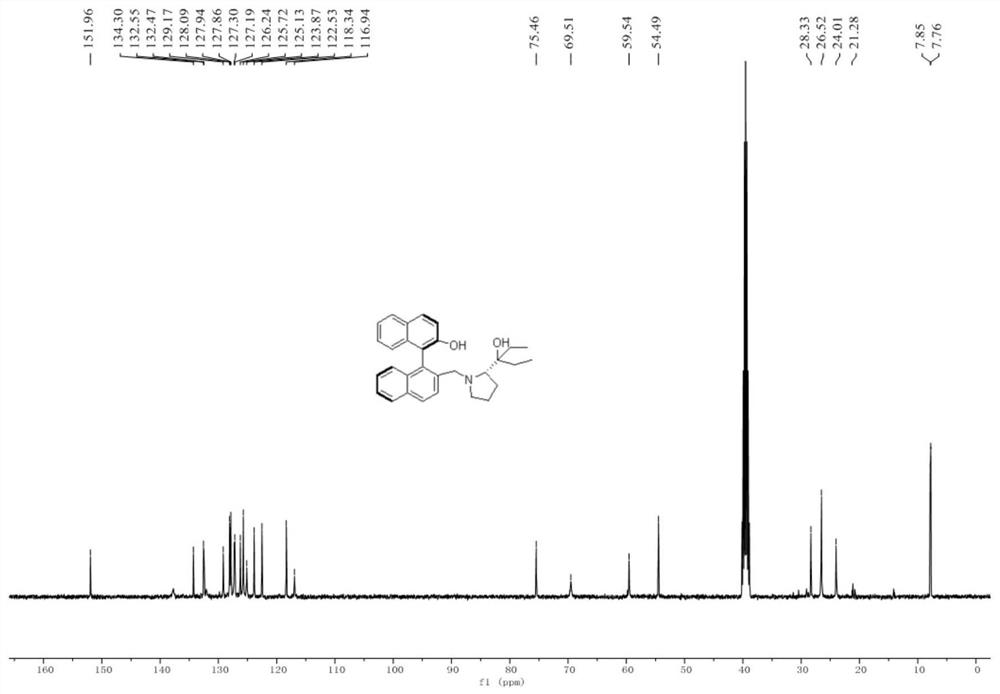
[0166] A novel chiral binaphthalene-aza five-membered ring ligand: (R a ,S)-2'-((2-(3-Hydroxypent-3-yl)pyrrolidin-1-yl)methyl)-1,1'-binaphth-2-ol, code L-1, The structural formula is as follows:
[0167]
[0168] Preparation of Compound V-1:
[0169]
[0170] Dissolve compound IV-0 (1 eq) and B3 (1.5 eq) in acetone, add potassium carbonate (6 eq) and sodium iodide (0.2 eq) to it, and react at room temperature for 24 h. After TLC detects that the reaction is complete, extract with ethyl acetate and water, wash with saturated brine, collect the upper organic phase, dry over anhydrous magnesium sulfate, remove the solvent under reduced pressure, and recrystallize from petroleum ether ethyl acetate to obtain a white solid product (compound V- 1), yield: 90%; 1H NMR (400MHz, DMSO) δ8.10(d, J=9.1Hz, 1H), 8.06–7.90(m, 4H), 7.63(d, J=9.1Hz, 1H), 7.41(t, J=7.4Hz ,1H),7.30(t,J=7.5Hz,1H),7.19(dd,J=15.3,7.8Hz,2H),6.91(d,J=8.5Hz,1H),6.75(d,J=8.5Hz ,1H),3.79(d,J=13.8Hz,1H),3.72(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com