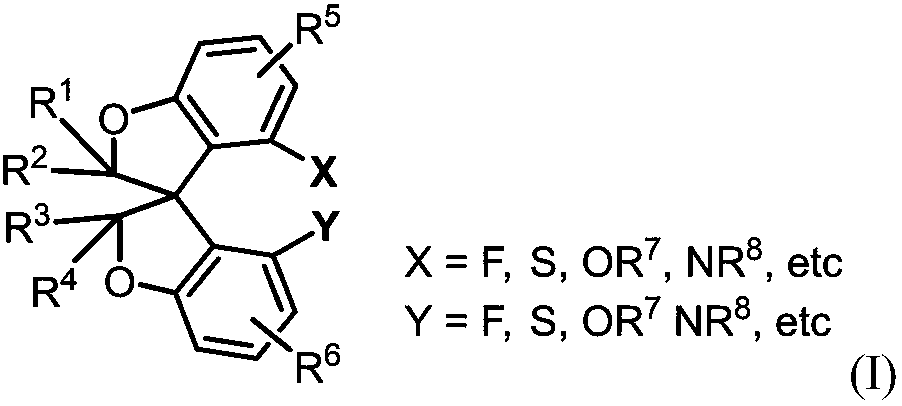
Oxaspiro compound and efficient synthesis and splitting method thereof
A ring compound, oxaspiro technology, applied in the field of a new type of oxaspiro compound and its synthesis and resolution, can solve the problem of insufficient number of spiro ring skeleton ligands, difficulty in spiro ring skeleton synthesis, limited modification, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
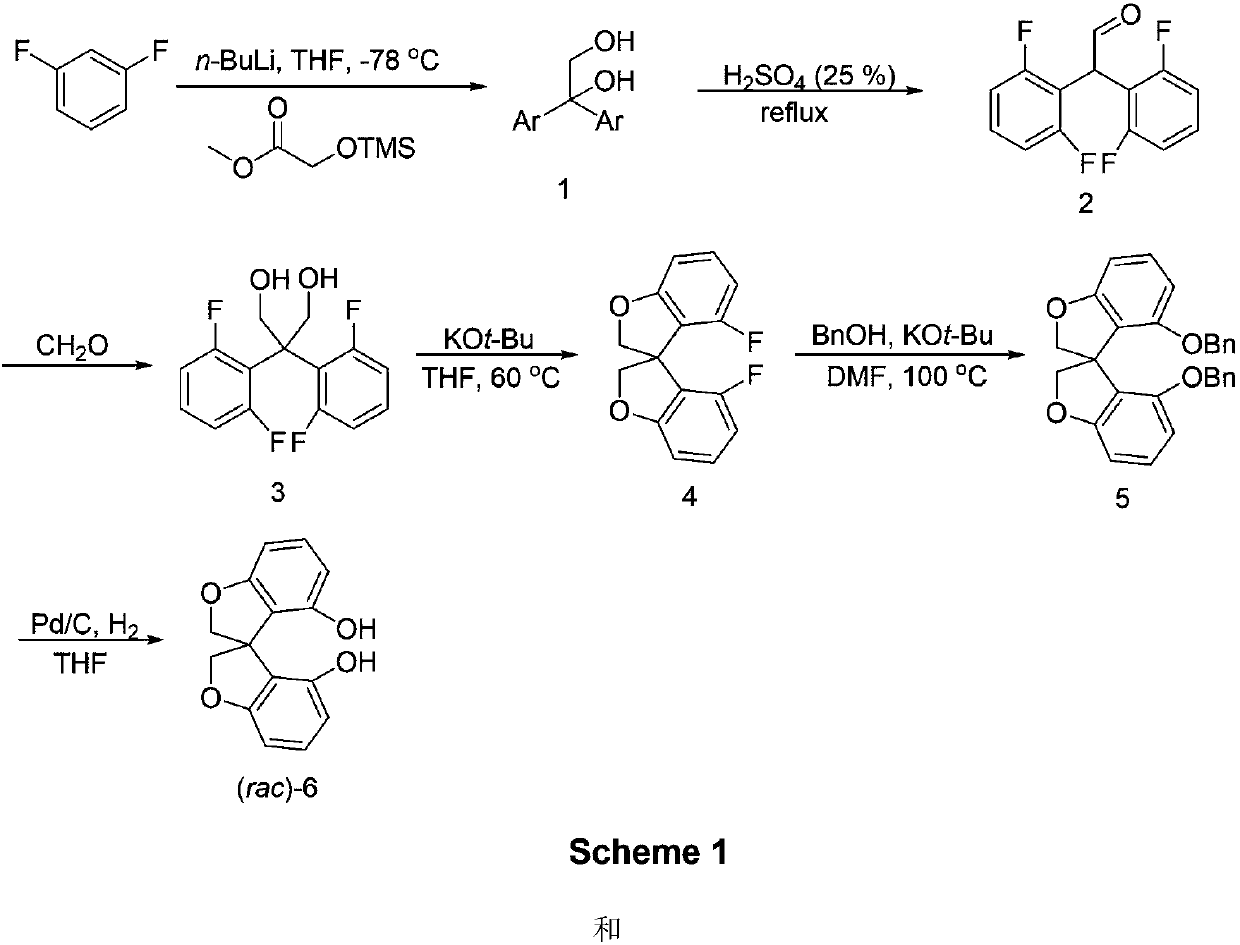
[0031] Synthesis of compound 6:
[0032]
[0033] 1,3-Difluorobenzene (35g, 306mmol) was dissolved in 150mL of anhydrous tetrahydrofuran, under Ar protection, at -78°C, n-butyllithium (123mL, 2.5M n-hexane solution) was added dropwise, and the addition was completed After that, it was stirred at -78°C for 1 h, and then methyl trimethylsiliconate (24.339 g, 150 mmol) was added slowly. After the addition was complete, the reaction was warmed to -30°C and stirred for a while and then warmed to room temperature. After the reaction was completed, dilute hydrochloric acid was added at low temperature to quench the reaction, and at the same time, the trimethylsilyl group was removed. The reaction mixture was extracted with ether and dichloromethane, and the organic phases were combined. The solvent was removed under reduced pressure to obtain the target product 1, which was directly subjected to the next reaction without purification.
[0034]
[0035] Compound 1 and 150 mL ...
Embodiment 2
[0050] Resolution of compound 6:
[0051]
[0052] In a 1000mL reaction flask, add rac-6 (51.2g, 200mmol), D-proline (11.5g, 100mmol), and then add 300mL ethyl acetate. The reaction mixture was stirred at reflux for 10 h. A large amount of white solids were precipitated, cooled to room temperature and filtered and collected white solid A; the mother liquor after filtration was desolvated under reduced pressure, and then D-proline (11.5g, 100mmol) was added again, the solvent was changed to acetonitrile, and the same under reflux conditions Under stirring for 10h, a large number of white solids were precipitated and cooled to room temperature. The white insoluble matter B was collected by filtration. The solvent was removed from the mother liquor under reduced pressure, and then the above resolution process was repeated. All the collected solid A was added to a mixed solvent of ethyl acetate and water for shaking, and the white insoluble matter gradually dissolved and dis...
Embodiment 3
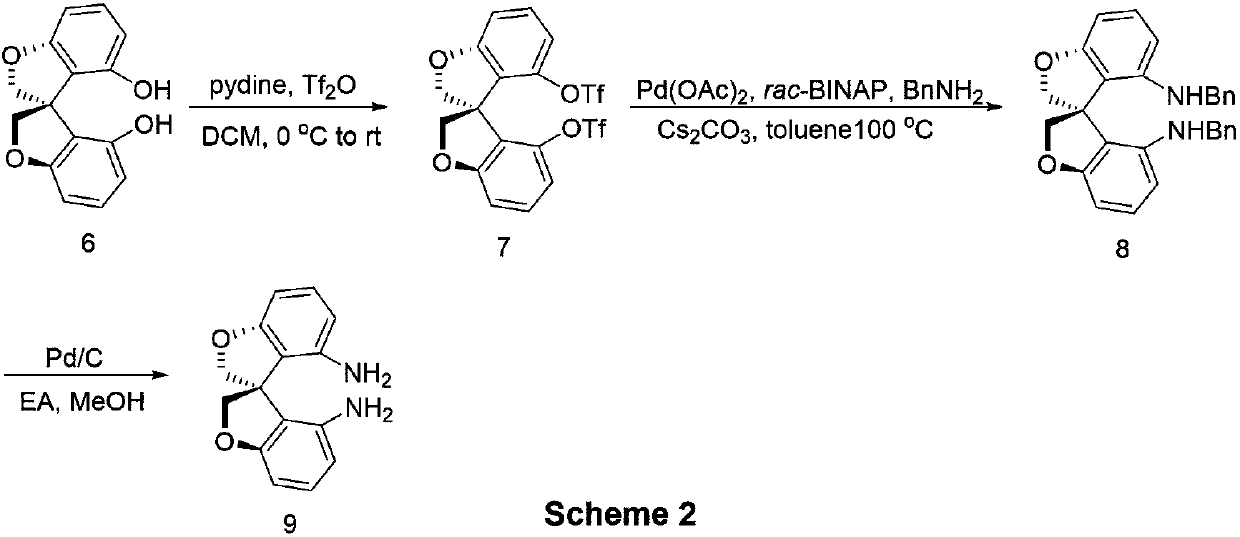
[0054] Synthesis of oxaspirocyclic diamines:
[0055]
[0056] N 2 To a 250 mL reaction flask was added (S)-6 (7.68 g, 30 mmol) followed by 150 mL of dry dichloromethane under atmosphere. Pyridine (6.0 mL, 100 mmol) was added under stirring at room temperature. After the reaction system was clarified, it was cooled to zero, and then Tf was added dropwise. 2 O (12.0mL, 70mmol), after the dropwise addition was completed, it was raised to room temperature and stirred for 1h. Water was added to quench the reaction. The reaction system was washed with dilute hydrochloric acid, the organic phase was desolvated under reduced pressure, and the product (S)-7 (15.6g, yield: 99%) could be obtained through column chromatography
[0057] 1 H NMR (500MHz, CDCl 3 )δ4.70 (d, J=10.0Hz, 2H, CH 2 ),4.87-4.90(m,2H,CH 2 ), 6.91-6.93 (m, 4H, Ar), 7.32 (dd, J 1 =8.5Hz,J 2 =8.0Hz,2H,Ar).
[0058]
[0059] N 2 Under atmosphere, add (S)-7 (2.60g, 4mmol), Pd(OAc) into the reaction tube...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com