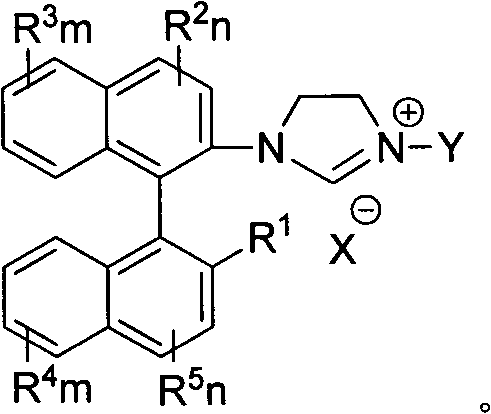
Axially chiral imidazole salt compound and preparation method thereof
A technology of axial chirality and imidazolium salt, which is applied in the field of synthesis of chiral catalysts, can solve the problems of chiral azacarbene synthesis, difficulty in synthesis, limitation of synthesis and application of new axial chiral azacarbene, etc., and achieve novel structure , Synthesis is simple and convenient, good effect of asymmetric selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 1,3-bis{2-[2'-methoxy-((R)-(+)-1,1'-naphthyl)]}imidazolium salt (I-1)
[0028]
[0029] Dissolve compound N1, N2-bis{(R)-(+)-2′-methoxy-[1,1′-naphthyl]}-1,2-ethylenediamine in MeOH / HCl=12ml / The acidification reaction was carried out in 1 ml of the mixed solution, which was stirred and reacted at room temperature for 1 hour. After it was cooled to room temperature, the solvent was distilled off under reduced pressure to obtain a yellow solid, and then the obtained yellow solid was mixed with P 2 o 5 Dry in a desiccator for 3 hours. Afterwards, the above yellow solid was dissolved in 5ml HC(OEt) under nitrogen protection 3 , react at 80°C for 1 hour. After it was cooled to room temperature, the precipitated solid was filtered and washed with Et 2 O washing. The obtained solid is the product after drying, with a yield of 67%. The melting point is 216°C.[α] 20 D =+80.0 (measurement concentration c=0.1, measurement solvent is CH 2 Cl 2 ). 1 H NM...
Embodiment 2
[0031]Preparation of 1,3-bis{2-[2'-hydroxyl-((R)-(+)-1,1'-naphthyl)]}imidazolium salt (V-2a)
[0032]
[0033] Taking compound N1, N2-bis{(R)-(+)-2'-methoxymethyl ether-[1,1'-naphthyl]}-1,2-ethylenediamine as raw material, its Dissolve in MeOH / HCl=12ml / 1ml, and reflux and stir at 80°C for 5 hours. After it was cooled to room temperature, the solvent was evaporated under reduced pressure, and then 2 o 5 Dry in a desiccator for 6 hours to obtain a yellow solid. Afterwards, the above yellow solid was dissolved in 5ml HC(OEt) under nitrogen protection 3 , reacted at 130°C for 6 hours, and after cooling to room temperature, the precipitated solid was filtered and washed with Et2O. The resulting solid was dried and the product yield was 70%. Its physical constants are: melting point is 221°C; [α]20D+31.0 (measured concentration c=0.1, measured solvent is DMSO). 1 H NMR (DMSO, 400MHz): δ3.65-3.70 (2H, m), 1.00 (3H, d, J=6.8Hz), 6.77 (2H, d, 8.4Hz), 7.10 (2H, d, 8.4Hz) , 7.2...
Embodiment 3
[0035] Preparation of 1,3-bis[2-((R)-(+)-1,1'-naphthyl)]imidazolium salt (I-3)
[0036]
[0037] The preparation method is the same as in Example 1, with compound N1, N2-two {(R)-(+)-[1,1'-naphthyl]}-1,2-ethylenediamine as raw material, the product yield is 61%. Its physical constants are: melting point is 200°C. [α]20D=+27.0 (measured concentration c=0.1, measured solvent is DMSO). 1 H NMR (DMSO, 400MHz): δ3.01 (2H, s), 3.72 (2H, s), 7.02-7.04 (4H, m), 7.27-7.76 (14H, m), 8.15-8.24 (8H, m) , 9.07 (1H, s).13C NMR (DMSO, 100MHz): δ51.4, 123.1, 124.8, 125.8, 126.0, 126.1, 126.6, 127.3, 127.4, 127.8, 128.3, 128.7, 129.2, 130.0, 131.5, 132.2, 132.3, 132.5, 132.7, 133.2, 158.4. Infrared spectrum IR (KBr) vmax 3422.2, 1614.0, 1589.2, 1504.8, 1265.4, 1007.0, 806.8, 784.0, 750.8cm-1. The calculated value of high resolution mass spectrum HRMS (ESI) ([M+H]+) is C43H31N2+1: 575.2482, and the actual measured value is 575.2481.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com