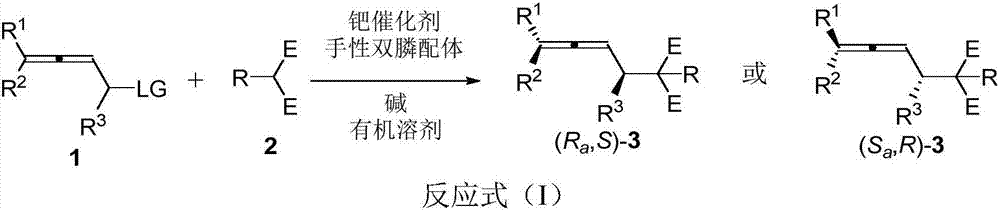
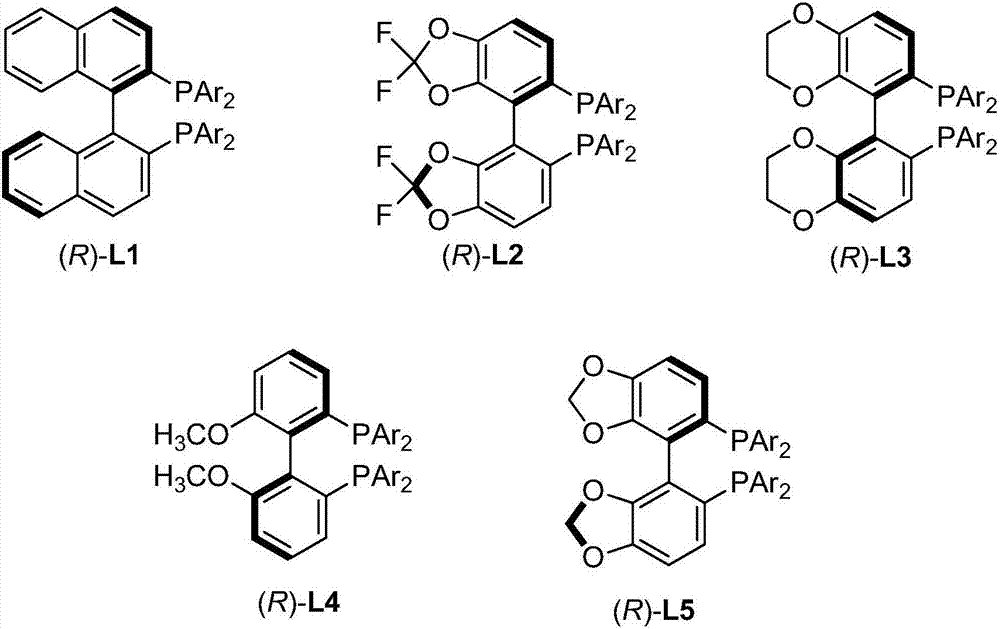
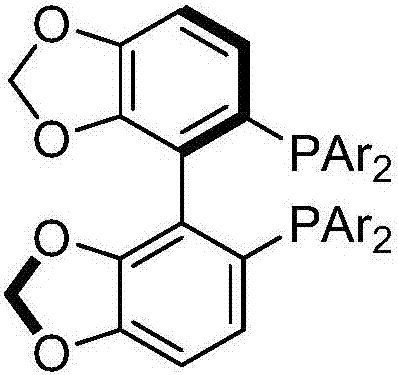
High optical activity allene compound combing axial chirality and central chirality, construction method and application thereof
An optically active, axial chiral technology, applied in the field of direct construction of highly optically active allene compounds with both axial chirality and central chirality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Wherein, equiv means equivalent weight, mol means mole, d.r. means diastereomer ratio, NMP means N-methylpyrrolidone, and ee means enantiomeric excess percentage.
[0046] In the glove box, sequentially add [Pd(π-cinnamyl)Cl] to a dry Schlenk reaction tube 2 (0.0134g, 0.025mmol), chiral bisphosphine ligand (R)-L5a (0.0709g, 0.06mmol), and K 2 CO 3 (0.2763g, 2mmol). Then NMP (5mL) was added under the protection of nitrogen, and the reaction tube was placed in an oil bath preheated to 30°C. After stirring for 30 minutes, the reaction tube was removed from the oil bath, and 2,3- Allenyl acetate (R a *,S*)-1a (0.2781 g, 1 mmol) with NMP (5 mL), diphenylsulfonylmethane 2a (0.6105 g, 2 mmol). The reaction tube was placed in a 30°C oil bath again, stirred, and the reaction was completed after 12 hours by thin layer chromatography (TLC). Ethyl acetate (30 mL) was added to dilute the reaction, and the resulting mixture was washed with brine (20 mL×3). The orga...
Embodiment 2
[0048]
[0049] Operation is with embodiment 1. [Pd(π-cinnamyl)Cl] 2 (0.0134g, 0.025mmol), (R)-L5a (0.0709g, 0.06mmol), K 2 CO 3 (0.2762g, 2mmol), NMP (5mL), (R a *,S*)-1b(0.3330g, 1 mmol) / NMP(5mL) and bis(phenylsulfonyl)methane(2a)(0.6105g, 2mmol) were reacted for 12 hours. Flash column chromatography (eluent: petroleum ether (30~60°C) / ethyl acetate=8 / 1) gave oily chiral allene product (R a ,S)-3ba(0.3990g,70%):98%ee(HPLC conditions:Chiralcel OZ-H column, n-hexane / i-PrOH=90 / 10,1.0mL / min,λ=214nm,t R (major) = 21.5min,t R (minor) = 48.7min); [α] D 20 =-78.5 (c=0.995, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ7.94(d, J=7.5Hz, 2H, ArH), 7.88(d, J=7.2Hz, 2H, ArH), 7.66(t, J=7.4Hz, 2H, ArH), 7.59-7.42(m , 4H, ArH), 5.91-5.72(m, 1H, =CH), 5.49-5.36(m, 1H, =CH), 5.12(td, J 1 =6.2Hz,J 2 =2.0Hz, 1H, =CH), 5.06-4.87(m, 2H, =CH 2 ), 4.66(d, J=1.8Hz, 1H, CH), 3.09-2.94(m, 1H, CH), 2.04(q, J=7.0Hz, 2H, CH 2 ),1.97-1.52(m,8H,5H from Cy,CH 2 , and CH),1.48-0.79(m,17H,5H from Cy ...
Embodiment 3
[0051]
[0052] Operation is with embodiment 1. [Pd(π-cinnamyl)Cl] 2 (0.0134g, 0.025mmol), (R)-L5a (0.0710g, 0.06mmol), K 2 CO 3 (0.2762g, 2mmol), NMP (5mL), (R a *,S*)-1c(0.2521g, 1 mmol) / NMP(5mL) and bis(phenylsulfonyl)methane(2a)(0.6105g, 2mmol) were reacted for 17 hours. Flash column chromatography (eluent: petroleum ether (30~60°C) / ethyl acetate=8 / 1) gave oily chiral allene product (R a ,S)-3ca(0.3428g,70%):97%ee(HPLC conditions:Chiralcel OZ-H column, n-hexane / i-PrOH=80 / 20,1.0mL / min,λ=214nm,t R (major) = 17.8min,t R (minor) = 31.2min); [α] D 20=-65.9 (c=1.05, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ 8.01-7.83(m,4H,ArH),7.73-7.59(m,2H,ArH),7.59-7.45(m,4H,ArH),5.44-5.32(m,1H,=CH),5.15(qd ,J 1 =6.6Hz,J 2 =2.1Hz,1H,=CH),4.67(d,J=1.5Hz,1H,CH),3.13-3.00(m,1H,CH),2.04-1.78(m,3H,1H from CH 2 and CH 2 ),1.77-1.61(m, 1H,1H from CH 2 ),1.50-1.00(m,12H,CH 2 ×6),0.97-0.79(m,6H,CH 3 ×2); 13 C NMR (75MHz, CDCl 3 )δ204.0, 140.1, 138.6, 134.3, 134.1, 129.4, 128.93, 128....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com