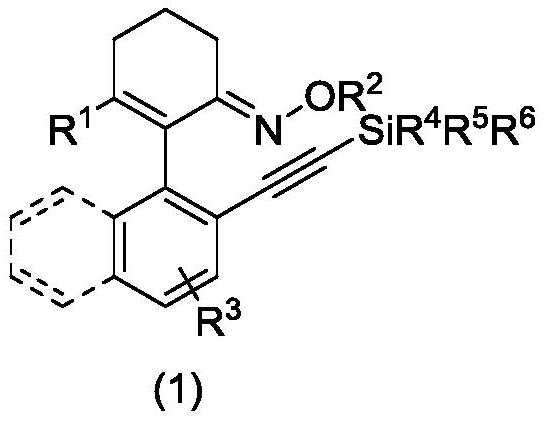
Axially chiral arylethynyl silane compound and preparation method thereof
An ethynyl silane and axial chirality technology, which is applied in the field of axial chiral arylethynyl silane compounds and their preparation, can solve the problems of complex substrates, high solvent melting points, and scarce sources of raw materials such as ligands, and achieves a simple catalytic system. , The effect of low production cost and easy industrialization promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (E)-3-Methyl-2-(2-((triisopropylsilyl)ethynyl)naphthalene-1-yl)cyclohex-2-en-1-one-O-methyloxime
[0034]
[0035] To a 25 mL reaction tube was added 3-methyl-2-naphthylcyclohex-2-en-1-one-O-methyloxime (0.13 g, 0.2 mmol), (2-bromoethynyl)triisopropyl Silane (0.16g, 0.6mmol), palladium acetate (0.005g, 10mol%), N-acetyl-L-alanine (0.0053g, 20mol%), silver carbonate (0.165g, 0.6mmol), then add methanol ( 2mL). The reaction tube was moved to a 40°C oil bath for 48 hours. The mixture was purified by flash column chromatography to obtain a yellow liquid (82 mg, 0.184 mmol), with a yield of 92%.
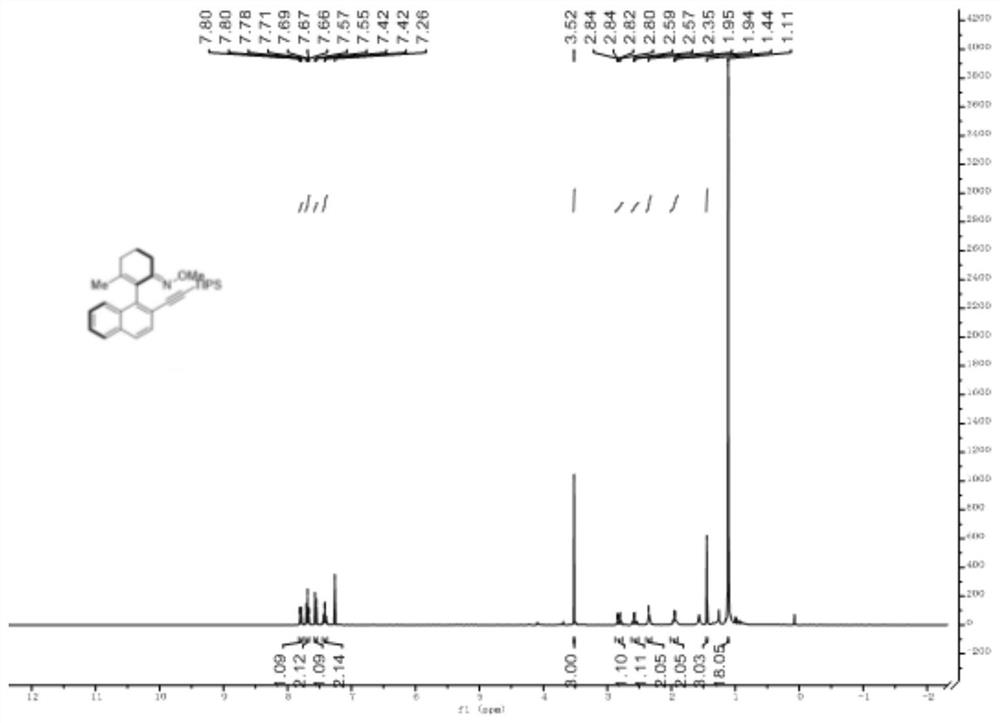
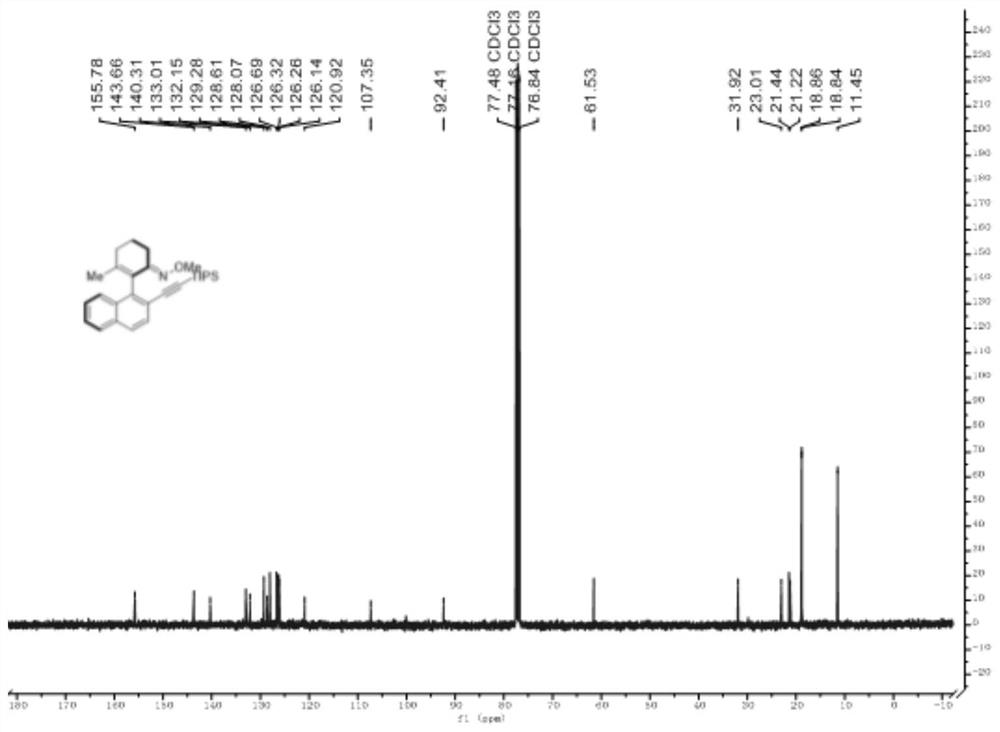
[0036] H NMR spectrum such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 )δ7.82-7.77(m,1H),7.68(dd,J=12.8, 4.9Hz,2H),7.56(d,J=8.4Hz,1H),7.43(m,2H),3.52(s,3H ),2.88-2.75(m,1H),2.59(m,1H),2.34(m,2H),1.95(m,2H),1.44(s,3H),1.11(d,J=2.1Hz,18H) .Carbon spectrum such as figure 2 as shown, 13 C NMR (100MHz, CDCl 3 )δ155.78(s),143.66(s),140.31(s),133.01(s),132.15(s),129.28(s), 1...
Embodiment 2
[0038] (E)-3-Methyl-2-(4-methyl-2-((triisopropylsilyl)ethynyl)naphthalene-1-yl)cyclohex-2-en-1-one-O- Methyl oxime
[0039]
[0040] To a 25 mL reaction tube was added 3-methyl-2-naphthylmethyl-2-cyclohexen-1-one-O-methyloxime (0.056 g, 0.2 mmol), (2-bromoethynyl)triisopropyl Silane (0.16g, 0.6mmol), palladium chloride (0.0018g, 5mol%), Boc-D-valine (0.0043g, 10mol%), silver trifluoromethanesulfonate (0.102g, 0.4mmol), Additional methanol (1 mL) was added. The reaction tube was moved to a 50°C oil bath for 40 hours. The mixture was purified by flash column chromatography to obtain white liquid (36.8 mg, 0.08 mmol), yield 40%.
[0041] 1 H NMR (400MHz, CDCl 3 )δ7.95(d,J=8.0Hz,1H),7.74-7.65(m,1H),7.51-7.37(m,3H),3.53(s,3H),2.82(m,1H),2.67(s ,3H),2.63-2.50(m,1H),2.34(m,2H),1.95(m,2H),1.45(s,3H),1.12(d,J=2.5Hz,18H). 13 C NMR (100MHz, CDCl 3 )δ155.89(s),143.69(s),138.62(s),132.85(s),132.40(s),132.24(s),129.72(s),128.75(s),126.92(s),125.99( s), 125.88(s), 124.24(s), 120....
Embodiment 3
[0043] (E)-2'-methoxy-6-methyl-6'((triisopropylsilyl)ethynyl)-4,5-dihydro-[1,1'-biphenyl]-2( 3H)-O-methyloxime
[0044]
[0045] To a 25 mL reaction tube was added 3-methyl-2-(2-methoxyphenyl)cyclohex-2-en-1-one-O-methyloxime (0.049 g, 0.2 mmol), (2-bromo Ethynyl) triisopropylsilane (0.16g, 0.4mmol), palladium tetraacetonitrile tetrafluoroborate (0.009g, 10mol%), Boc-L-isoleucine (0.0093g, 20mol%), silver carbonate (0.11 g, 0.4 mmol), and tetrahydrofuran (2 mL) was added. The reaction tube was moved to a 60°C oil bath for 36 hours. The mixture was purified by flash column chromatography to obtain white liquid (32.4 mg, 0.076 mmol), yield 38%.
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.16(m,2H),6.87(dd,J=7.9,1.3Hz,1H),3.74(s,3H),3.66(s,3H),2.68-2.52(m,2H),2.33-2.16 (m,2H),1.89-1.77(m,2H),1.56(s,3H),1.08(d,J=2.7Hz,18H). 13 C NMR (100MHz, CDCl 3 )δ157.18(s),155.63(s),142.54(s),130.81(s),127.50(s),126.75(s),125.25(s),125.03(s),111.61(s),106.51( s), 91.41(s), 61.48(s), 56.32(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com