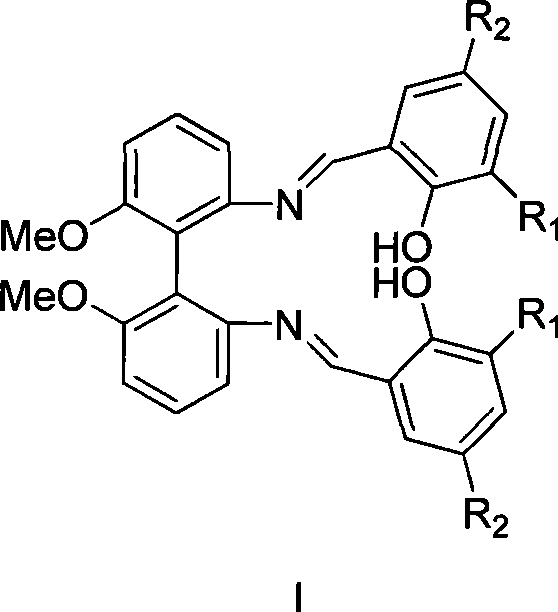
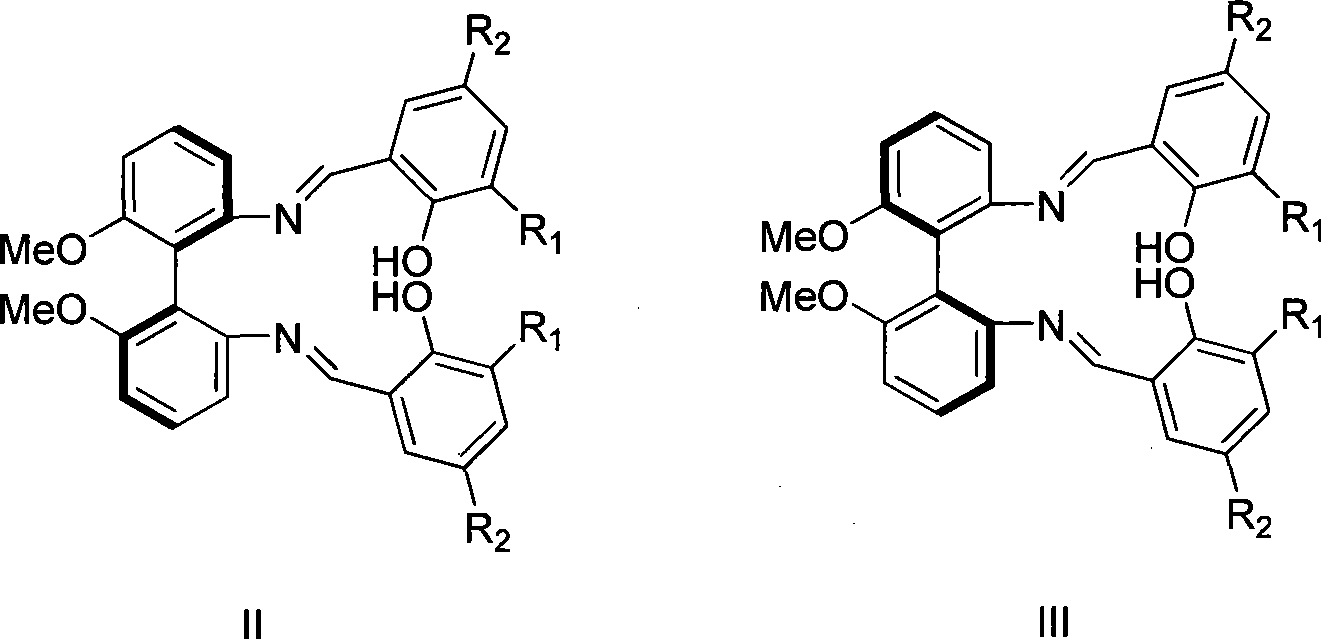
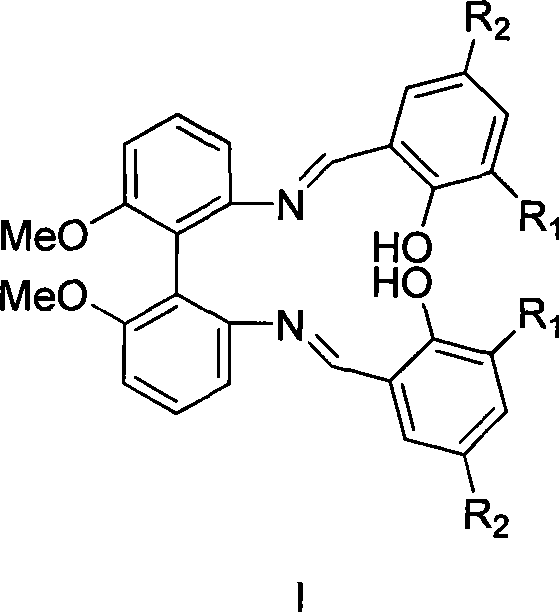
Axial chirality bis-schiff base-containing ligand
A bi-Schiff base and axial chiral technology, which is applied in the field of compounds in the field of chemical technology, can solve the problems of large steric hindrance and limit the rotation angle of biphenyl, etc., and achieve the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Preparation of compound II from compound IV (R 1 R 2 = Tert-butyl)
[0022] Compound IV (1.0 g, 4.1 mmol) and 3,5-di-tert-butyl salicylaldehyde (1.9 g, 8.2 mmol) were refluxed in ethanol for four hours. The solution produced a large amount of yellow solid. After the solvent was removed by filtration, the yellow solid was washed with cold ethanol and dried to obtain product II (2.38 g, 86%).
[0023] 1 H NMR(400MHz, CDCl 3 ) 13.07 (s, 2H, OH), 8.46 (s, 2H, CH=N), 7.39-7.08 (m, 10H, ArH), 3.81 (s, 6H, OCH 3 ), 1.37 (s, 18H), 1.21 (s, 18H).
Embodiment 2
[0025] (1) Preparation of compound III from compound V (R 1 R 2 = Tert-butyl)
[0026] Compound V (2.0 g, 8.2 mmol) and 3,5-di-tert-butyl salicylaldehyde (3.8 g, 16.4 mmol) were refluxed in ethanol for four hours. The solution produced a large amount of yellow solid. The solvent was removed by filtration, and the remaining yellow solid was washed with cold ethanol and dried to obtain product III (4.7 g, 85%).
[0027] 1 H NMR(400MHz, CDCl 3 ) 13.07 (s, 2H, OH), 8.46 (s, 2H, CH=N), 7.39-7.08 (m, 10H, ArH), 3.81 (s, 6H, OCH 3 ), 1.37 (s, 18H), 1.21 (s, 18H).
Embodiment 3
[0029] (1) Preparation of compound II from compound IV (R 1 R 2 =Chlorine)
[0030] Compound IV (1.0 g, 4.1 mmol) and 3,5-dichlorosalicylic aldehyde (1.6 g, 8.2 mmol) were refluxed in ethanol for six hours. After the reaction, the solvent was removed by filtration, and the remaining solid was washed with cold ethanol and dried to obtain product II (2.4 g, 85%).
[0031] 1 H NMR(400MHz, CDCl 3 ) 13.01 (s, 2H, OH), 8.46 (s, 2H, CH=N), 7.45-7.11 (m, 10H, ArH), 3.75 (s, 6H, OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com