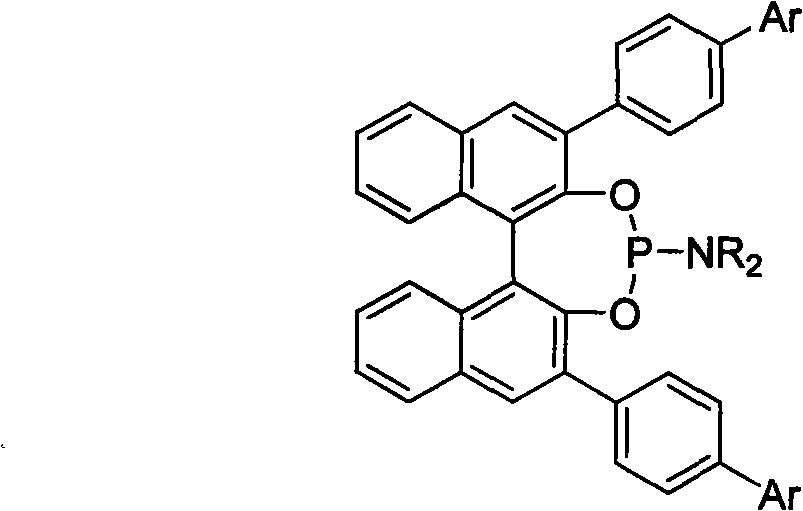
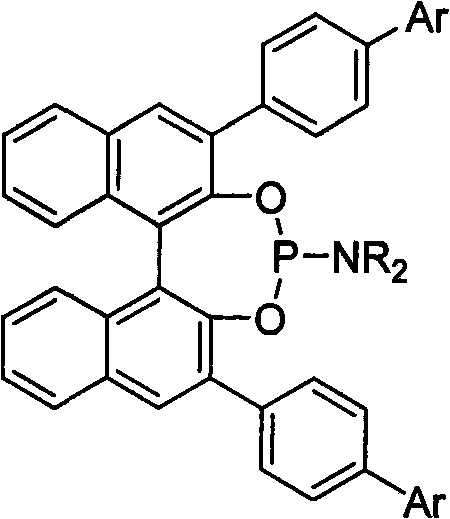
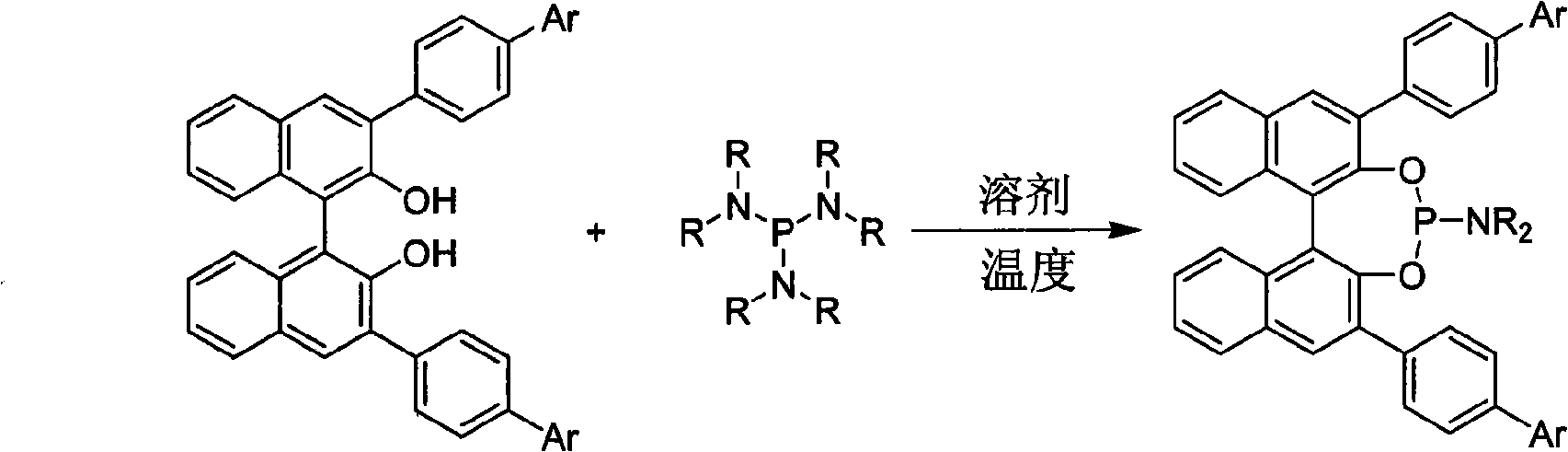
3, 3'-position biaryl group binaphthyl shaft chiral phosphoramidite ligand and preparation method thereof
A phosphoramidite and axial chiral technology, applied in the field of new chiral phosphoramidite ligands, can solve the problem of low enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1: Ligand (R)-3,3'-bis(4-biphenyl)-2,2'-binaphthol-N, the preparation of N-dimethylphosphoramidite:
[0022] Under the protection of argon, (R) 3,3'-4-phenyl-phenyl-1,1'-binaphthyl-2,2'-diol 591mg (1.0mmol), hexamethylphosphorous Add 163mg (1.0mmol) of triamine into a 20mL Schlenk reaction flask, and add 5mL of dry toluene, heat at 110°C for 12 hours, and the reaction is complete as detected by thin-layer chromatography. The reaction mixture was evaporated to remove the solvent, and separated by flash silica gel column (petroleum ether / ethyl acetate=20 / 1 as the eluent) to obtain the target product, 560 mg, with a yield of 85.0%. 1 H NMR (500MHz, CDCl 3 ), δppm: 2.48 (s, 6H, 2×CH 3 ), 7.25(s, 8H, Ar-H), 7.30(m, 2H, Ar-H), 7.35(m, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H) , 7.48(d, J=8.5Hz, 2H, Ar-H), 7.63(d, J=8.5Hz, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H), 7.80 (m, 2H, Ar-H), 8.40 (d, J = 8.5 Hz, 2H, Ar-H).
[0023]
[0024] The ligand is coordinated with copp...
example 2
[0025] Example 2: Ligand (S)-3,3'-bis(4-biphenyl)-2,2'-binaphthol-N, the preparation of N-dimethylphosphoramidite:
[0026] Method similar to Example 1, with (S) 3,3'-4-phenyl-phenyl-1,1'-binaphthyl-2,2'-diphenol and hexamethylphosphorous triamide as raw materials , with toluene as the solvent, the reaction temperature was 110°C, and the corresponding ligand was synthesized with a yield of 83%. 1 H NMR (500MHz, CDCl 3 ), δppm: 2.47 (s, 6H, 2×CH 3 ), 7.25(s, 8H, Ar-H), 7.32(m, 2H, Ar-H), 7.38(m, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H) , 7.48(d, J=8.5Hz, 2H, Ar-H), 7.65(d, J=8.5Hz, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H), 7.81 (m, 2H, Ar-H), 8.41 (d, J = 8.5 Hz, 2H, Ar-H).
[0027]
[0028] The ligand is coordinated with copper trifluoromethanesulfonate to catalyze the conjugated addition reaction of diethylzinc and α-ylidene malonate to obtain the addition product with a yield of 80% and enantioselectivity The ee value reaches 85%.
example 3
[0029] Example 3: Ligand (S)-3,3'-bis(4-biphenyl)-2,2'-binaphthol-N, the preparation of N-diethylphosphoramidite:
[0030] Method similar to example 1, with (S) 3,3'-4-phenyl-phenyl-1,1'-binaphthyl-2,2'-diphenol and hexaethylphosphorous triamide as raw materials , using benzene as a solvent, the reaction temperature is 100°C, and the corresponding ligand is synthesized with a yield of 76%. 1 H NMR (500MHz, CDCl 3 ), δppm: 1.02(t, 6H, 2×CH 3 ), 2.60 (dd, J=8.5Hz, J=15.5Hz, 4H, 2×CH 2 ), 7.25(s, 8H, Ar-H), 7.32(m, 2H, Ar-H), 7.38(m, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H) , 7.48(d, J=8.5Hz, 2H, Ar-H), 7.65(d, J=8.5Hz, 2H, Ar-H), 7.45(d, J=8.5Hz, 4H, Ar-H), 7.81 (m, 2H, Ar-H), 8.41 (d, J = 8.5 Hz, 2H, Ar-H).
[0031]
[0032] The ligand is coordinated with copper trifluoromethanesulfonate to catalyze the conjugated addition reaction of diethyl zinc and α-ylidene-β-carbonyl carboxylate to obtain the addition product with a yield of 92%. The enantioselectivity ee value reach...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com