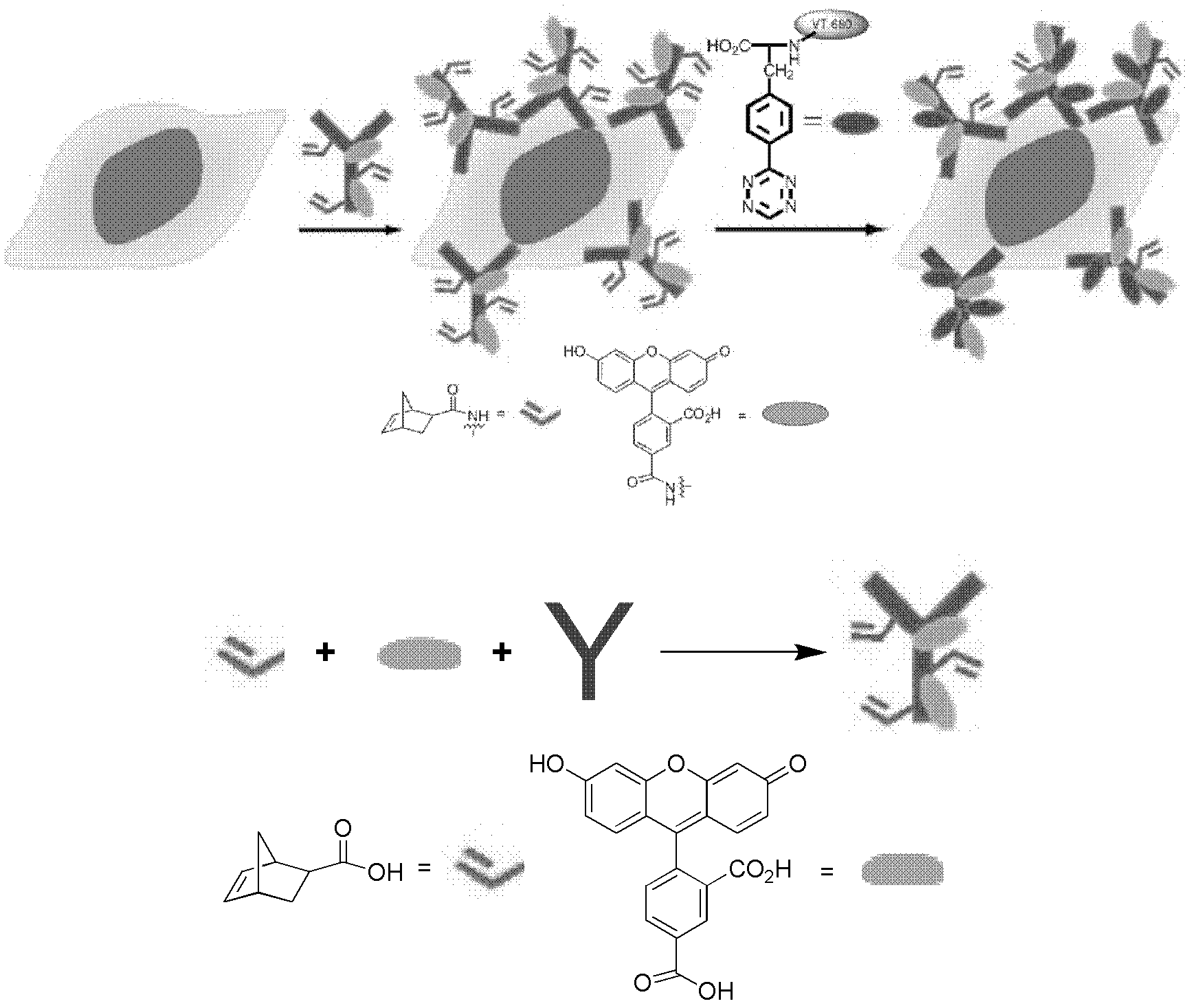
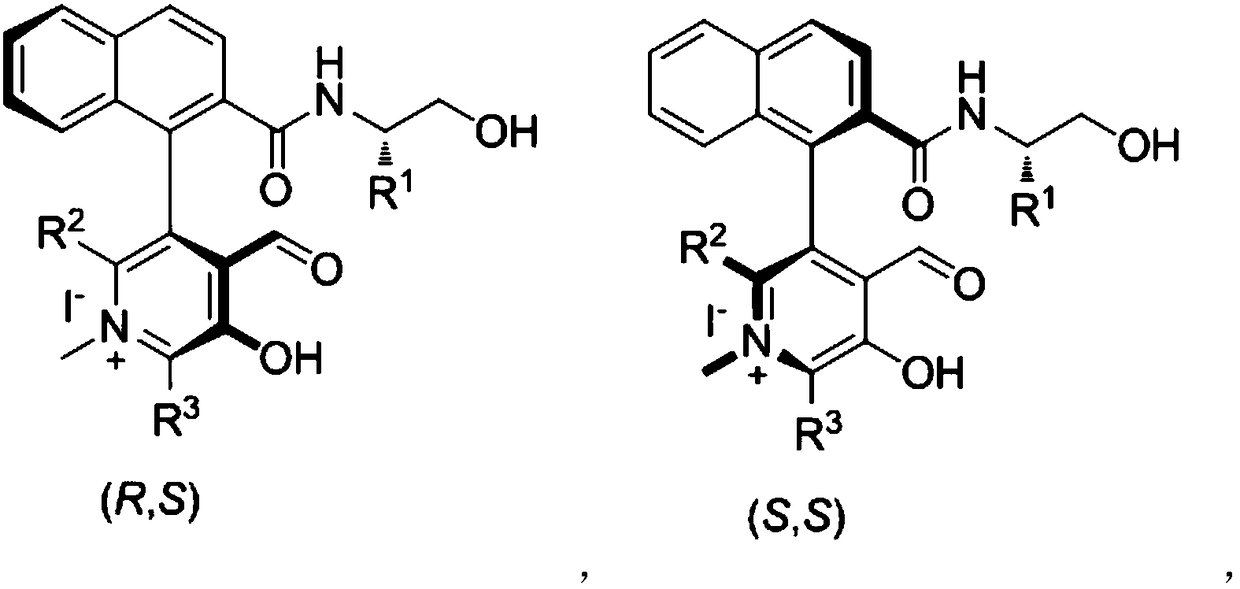
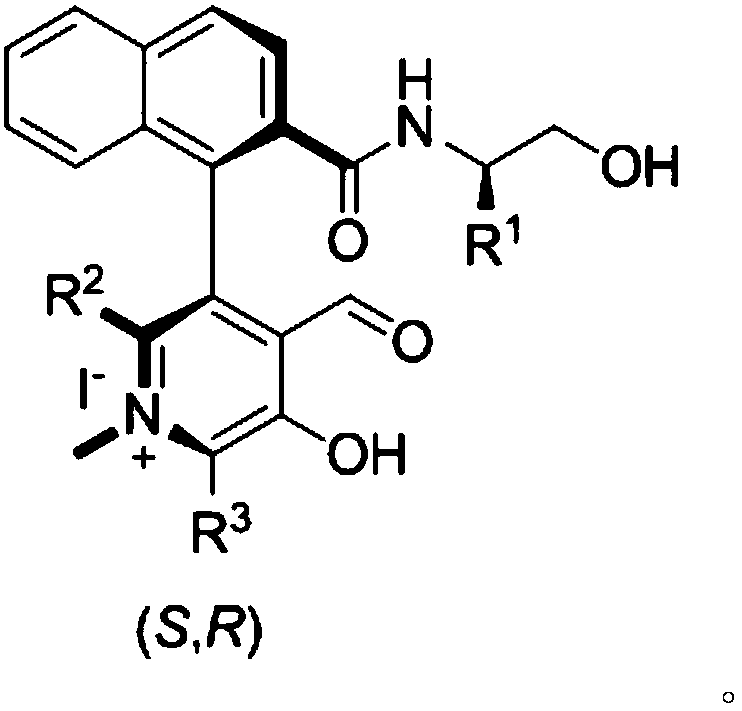
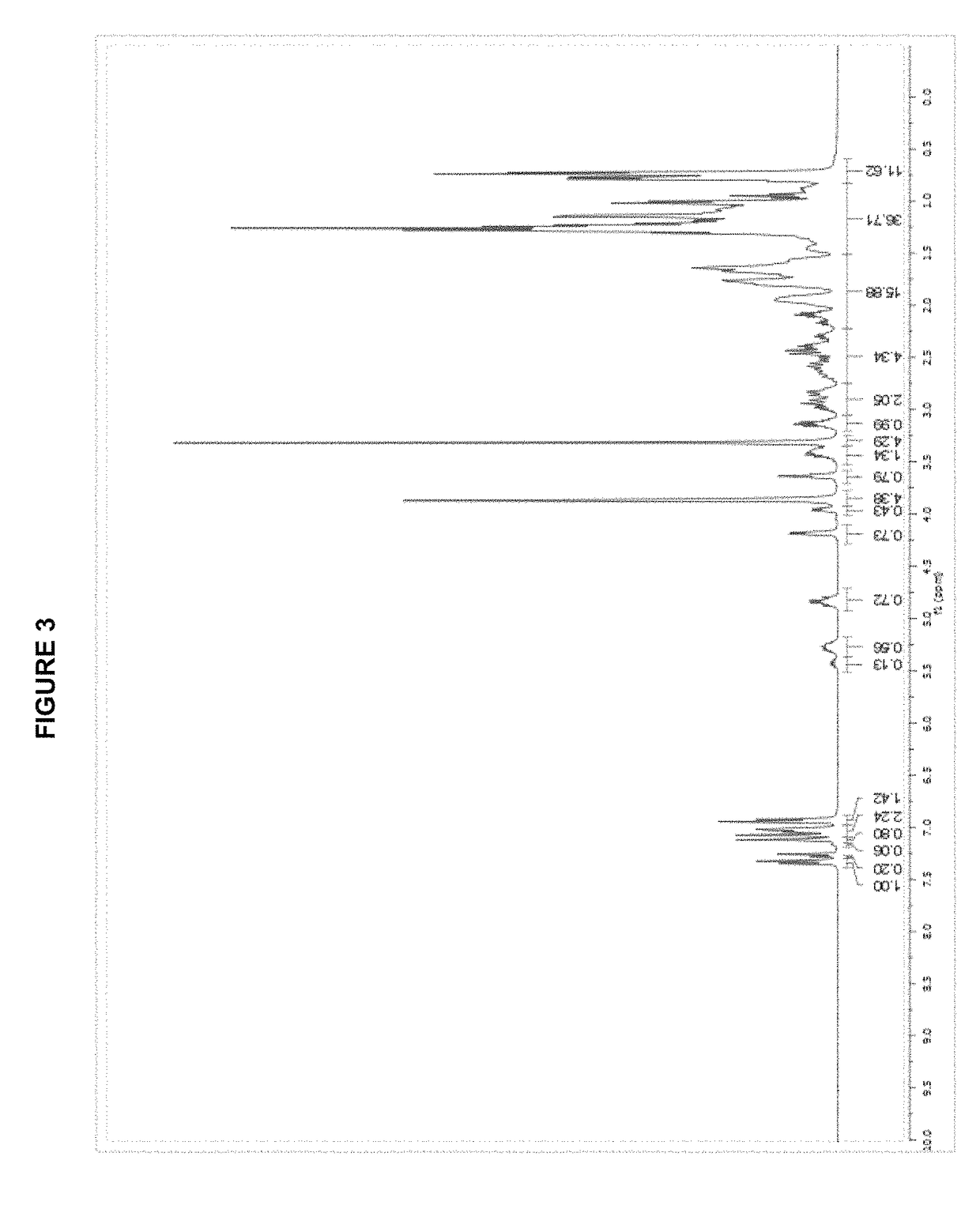
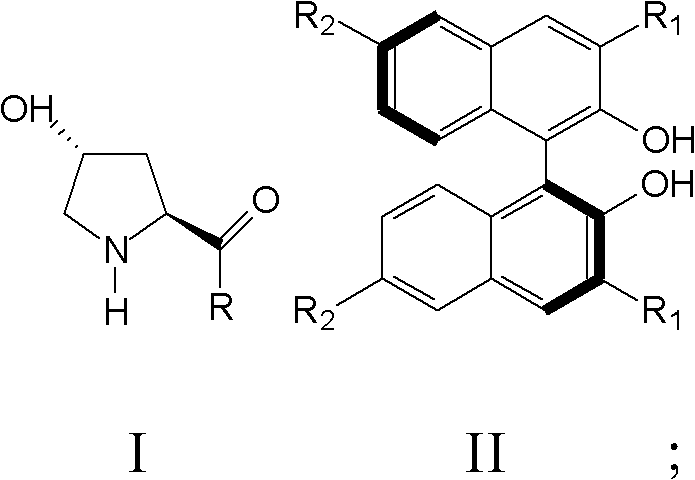
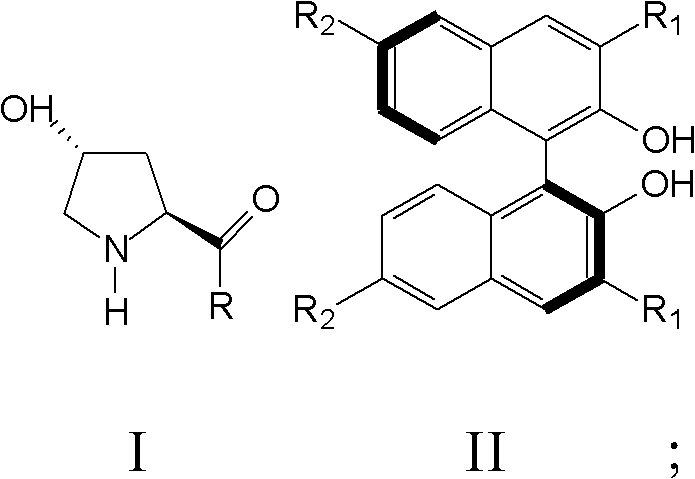
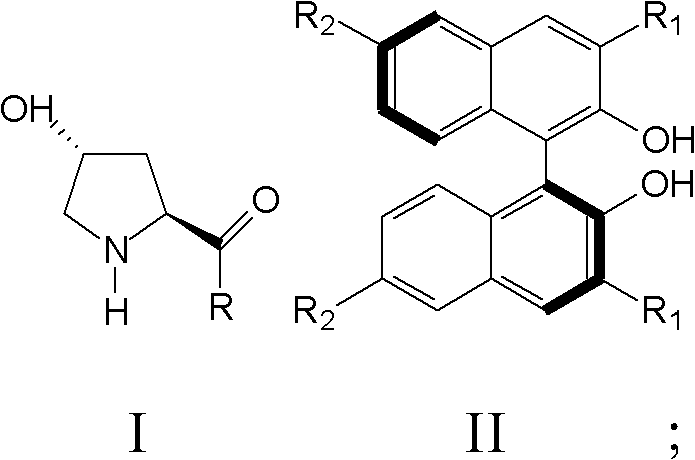
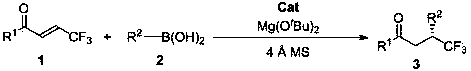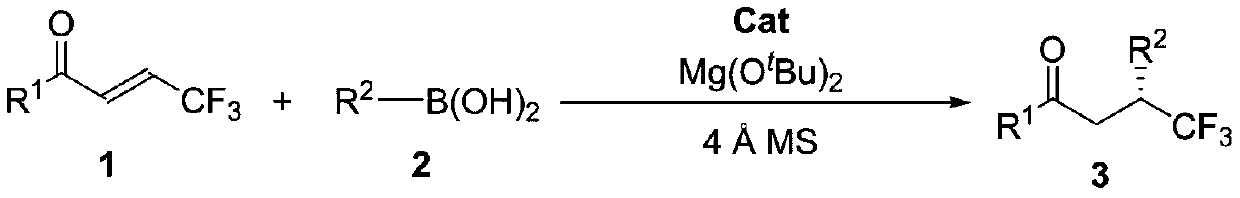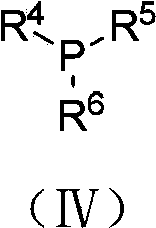Patents
Literature
110results about "Organic addition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Continuous process for the alkylation of hydrocarbons
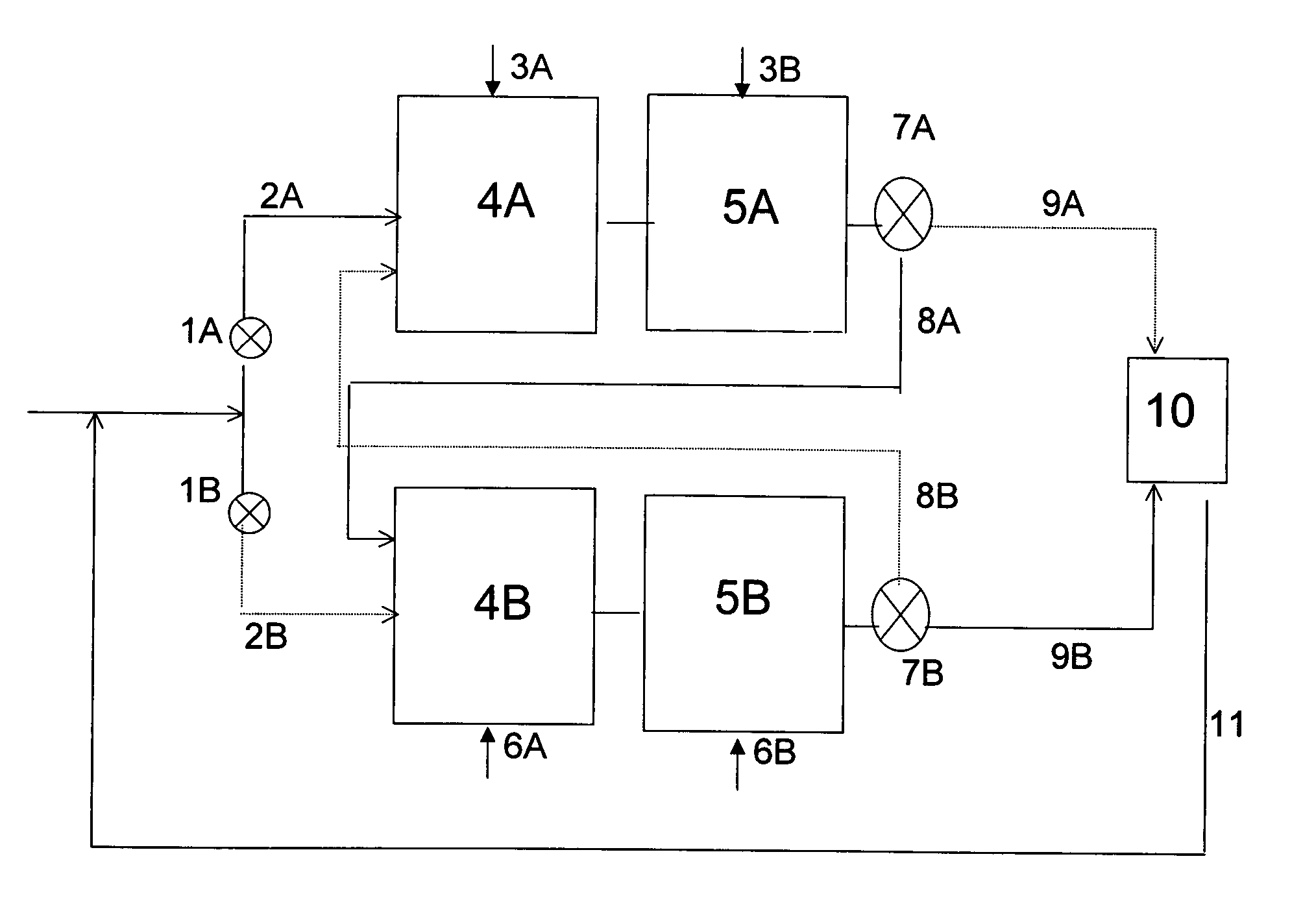
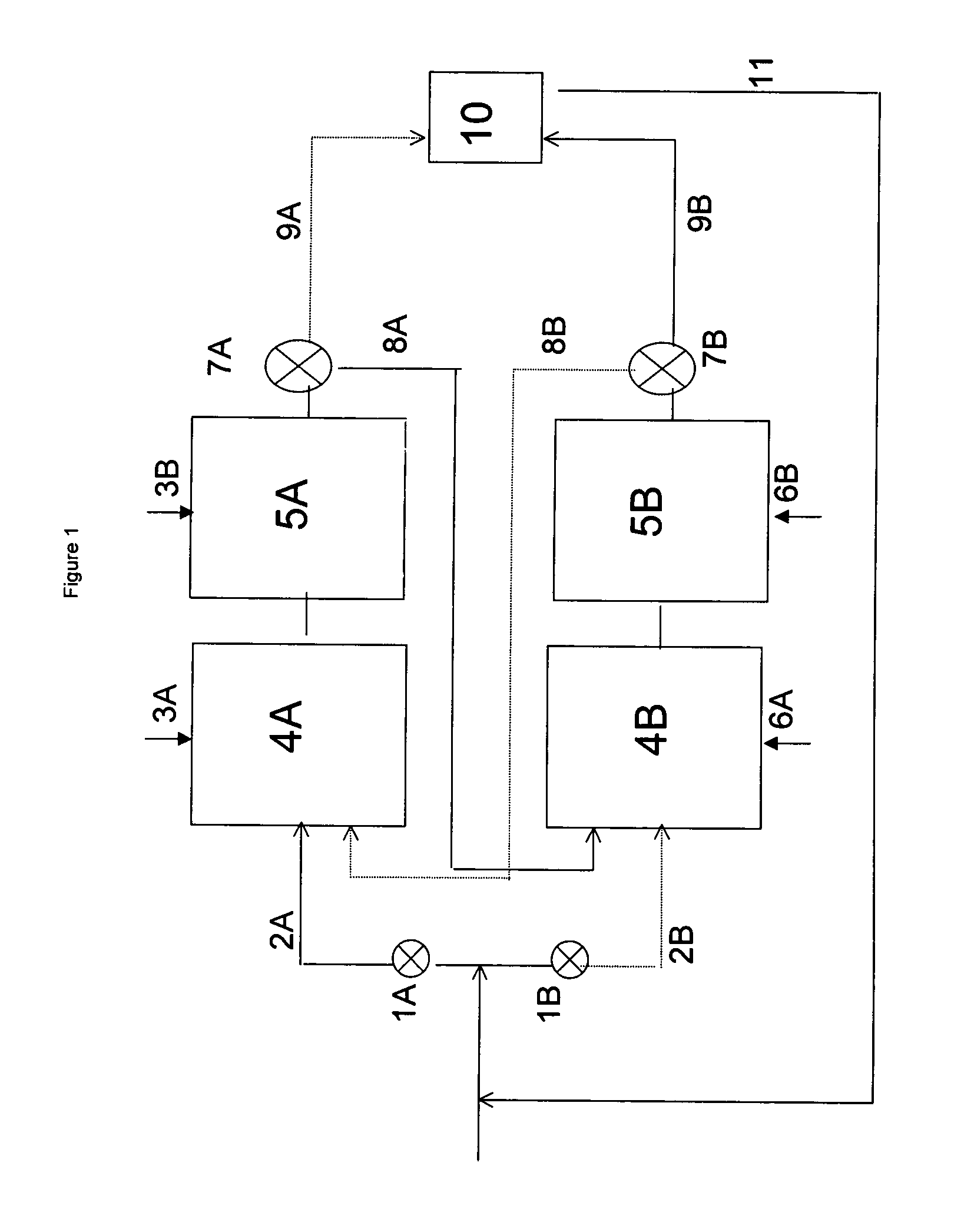
A continuous alkylation process performed in an apparatus comprising a series of at least two zone A reactors and a series of at least two zone B reactors, in which the zone A reactors and the zone B reactors cycle between alkylation mode and mild regeneration mode, and wherein the alkylation mode comprises introducing an alkylation agent into a first reactor of the zone through which the alkylatable compound passes, reacting a portion of the alkylatable compound with a portion of the alkylation agent to produce a product stream, and performing this operation at least once more in a downstream reactor in the same zone employing, instead of alkylatable compound, a stream comprising the product stream.
Owner:ALBEMARLE NETHERLANDS BV
Surface modified carbonaceous materials
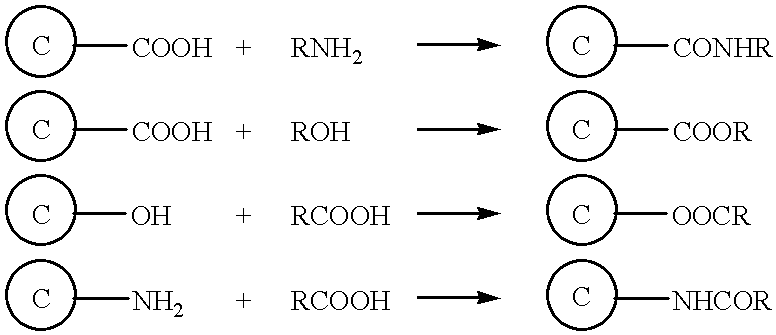
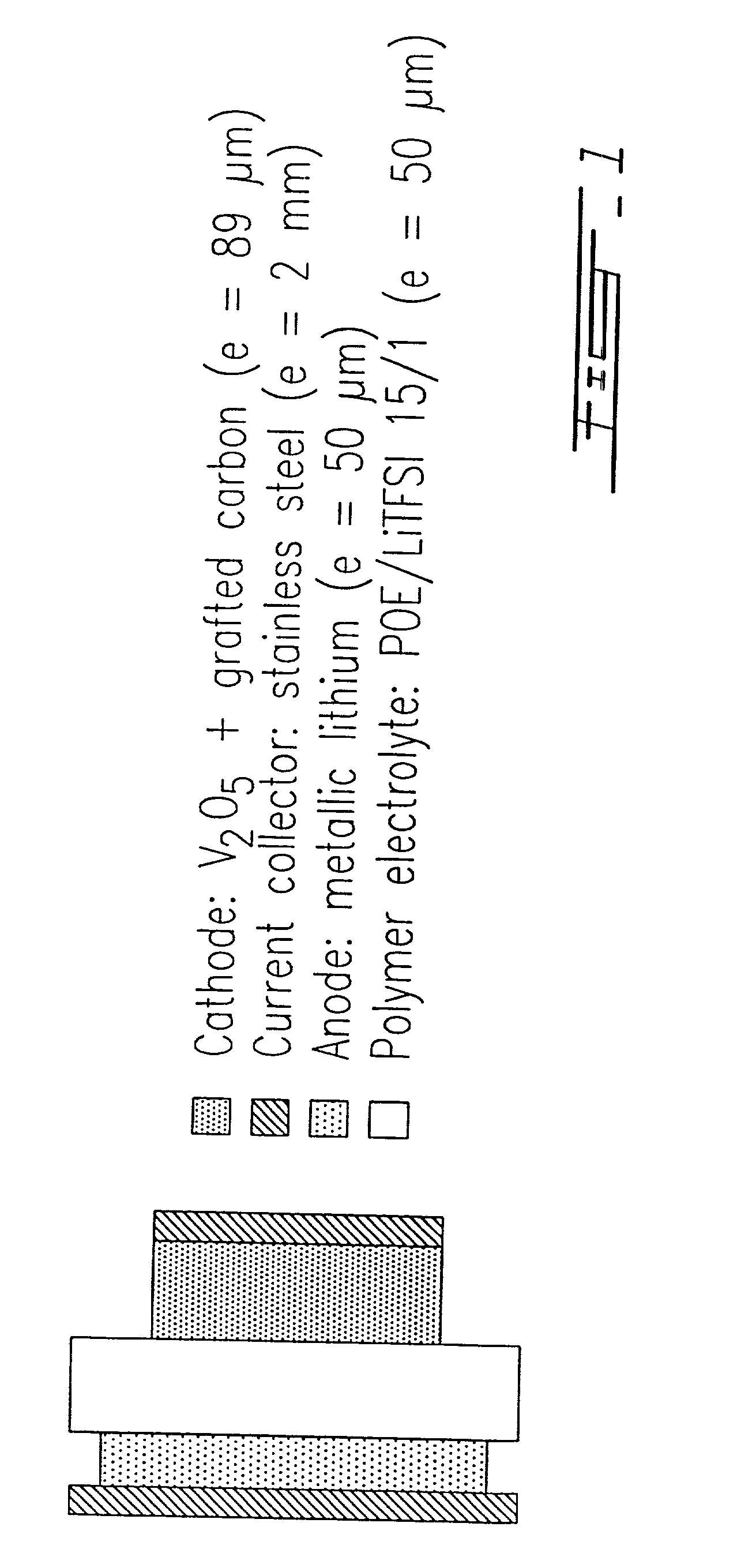
Grafting a polymer at the surface of a carbonated material containing carboxyl, amine and / or hydroxyl functions at its surface. This material is suspended in a solution comprising the polymer to be grafted, which includes a carboxyl, amine and / or hydroxyl function, the solution also comprising a solvent of the polymer. This is followed by a treatment causing dehydration into a carboxyl function, an amide and / or hydroxyl function and the polymer is thus grafted on the carbonated material by means of ester or amide bonds. Utilization in the cathode or anode of an electrochemical generator, in a polymer material with low polarity, in an ink, and as a conductive deposit on flexible plastic used as electrical contact, electromagnetic protection and antistatic protection.
Owner:HYDRO QUEBEC CORP +1
Surface modified carbonaceous materials
Grafting a polymer at the surface of a carbonated material containing carboxyl amine and / or hydroxyl functions at its surface. This material is suspended in a solution comprising the polymer to be grafted, which includes a carboxyl amine and / or hydroxyl function, the solution also comprising a solvent of the polymer. This is followed by a treatment causing dehydration into a carboxyl function, an amide and / or hydroxyl function and the polymer is thus grafted on the carbonated material by means of ester or amide bonds. Utilization in the cathode or anode of an electrochemical generator, in a polymer material with low polarity, in an ink, and as a conductive deposit on flexible plastic used as electrical contact, electromagnetic protection and antistatic protection.
Owner:HYDRO QUEBEC CORP +1
Acylation reactions of aromatic substrates
InactiveUS6630606B2Increase ratingsHigh selectivityCarbonyl compound preparation by condensationHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferProduct formation
Method of performing alkylation or acylation reactions of aromatic substrates under supercritical or near-critical reaction conditions. In particular, a method of performing Friedel-Crafts alkylation or acylation reactions is disclosed under those conditions. Friedel-Crafts reactions may be effected using a heterogeneous catalyst in a continuous flow reactor containing a super-critical or near-critical reaction medium. Selectivity of product formation can be achieved by varying one or more of the temperature, pressure, catalyst, flow rates and also by varying the ratios of aromatic substrate to acylating or alkylating agent.
Owner:THOMAS SWAN & CO LTD +1
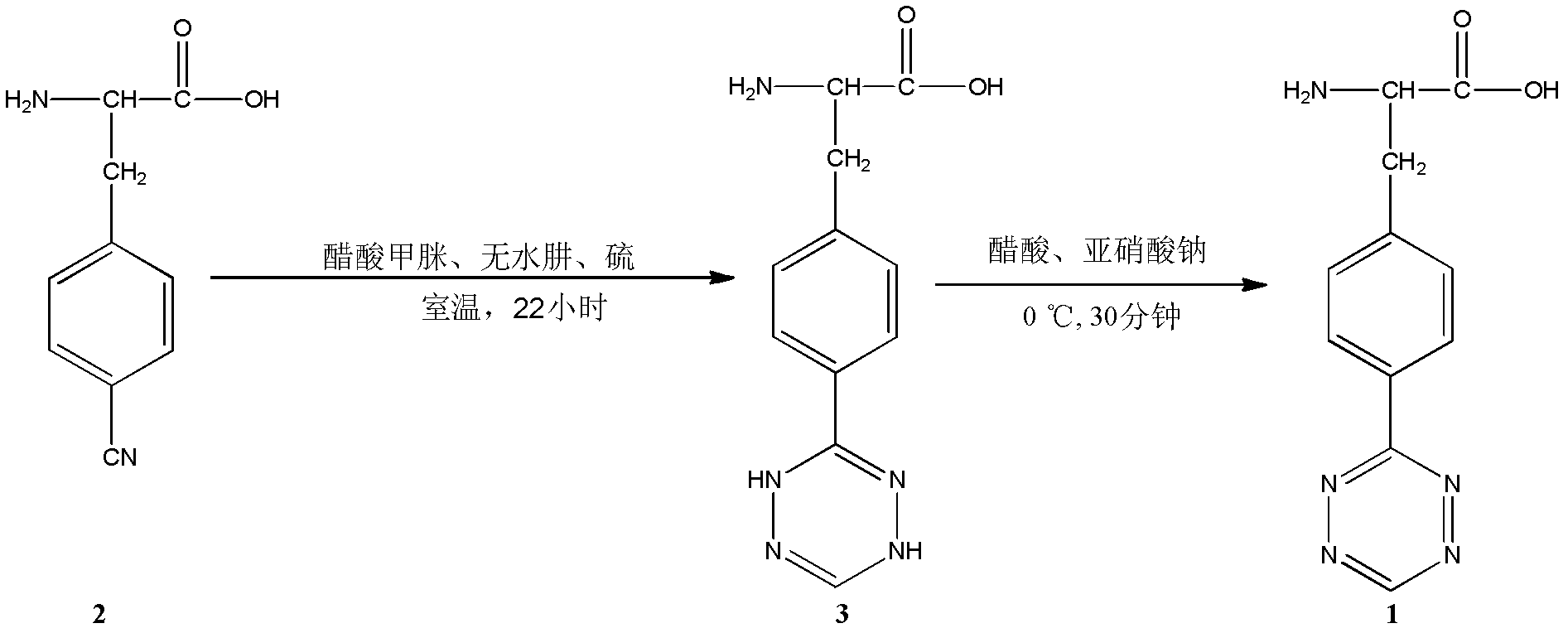
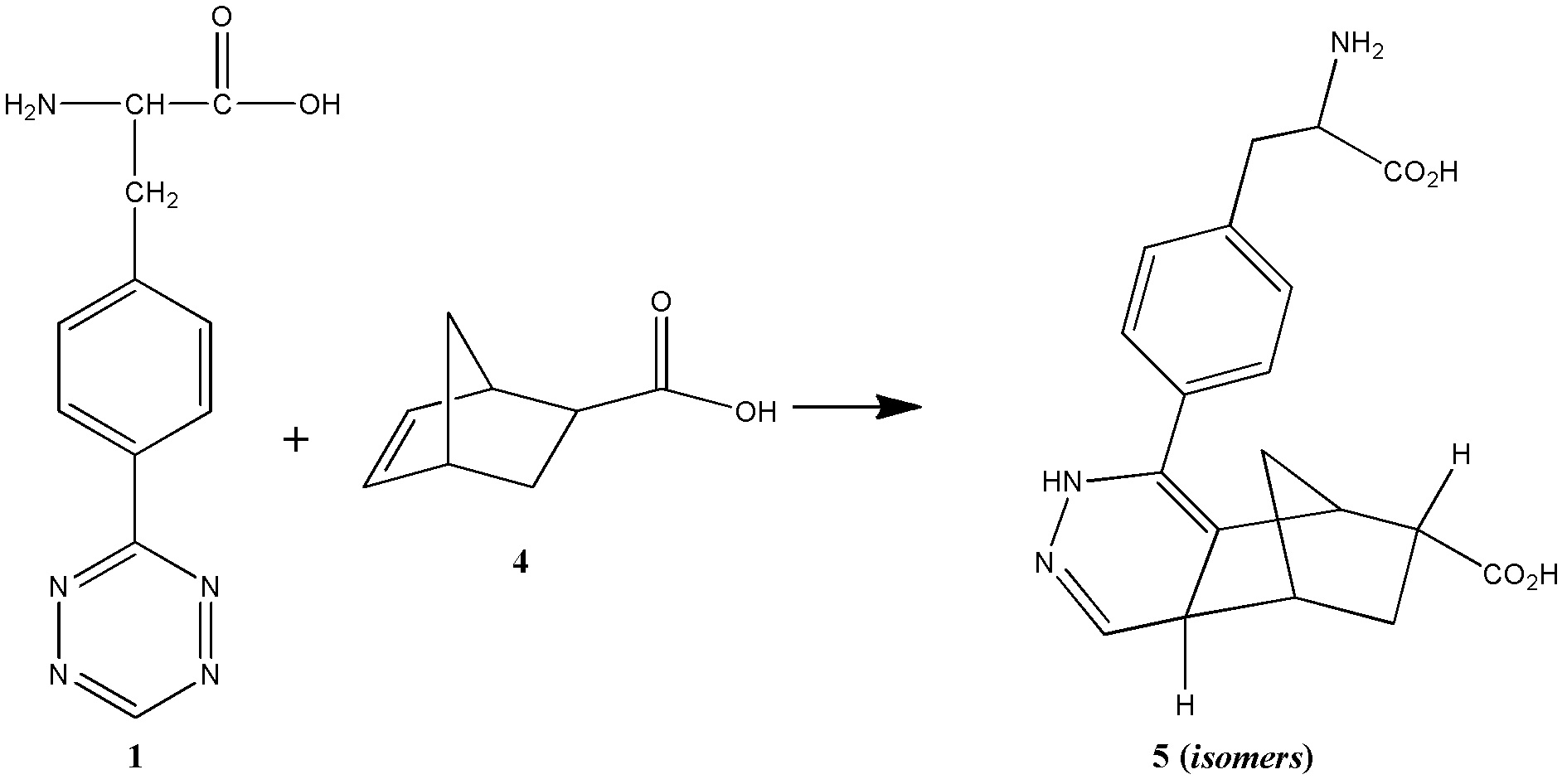
Novel compound L-4-terazine-phenylalanine, preparation method and application thereof
InactiveCN102627615AGood medicineHigh yieldPeptide preparation methodsOrganic additionFormamidine acetateProtein molecules
The invention discloses a novel amino acid derivative L-4-terazine-phenylalanine (3-(4-(1,2,4,5-tetrazin-3-yl) phenyl)-2-aminopropanoic acid). L-4-cyan-phenylalanine is used as an initial reactant and reacts with formamidine acetate and anhydrous hydrazine under catalysis of sulfur; then the reactants are oxidized by sodium nitrite to generate L-4-1,2,4,5-terazine-phenylalanine. The L-4-1, 2, 4, 5-terazine-phenylalanine is integrated into the biologically active peptide and protein molecules as phenylalanine / tyrosine analogue, and can be applied to on biological orthogonal field based on inverse electronic Diels-Alder reaction as a biomarker; meanwhile, the L-4-1,2,4,5-terazine-phenylalaninethe can be introduced into biologically active peptide as phenylalanine / tyrosine analogue through solid phase polypeptide synthesis method and conduct pharmacological evaluation, so as to improve drug property of certain biologically active peptide.
Owner:LANZHOU UNIVERSITY
Method for reducing alkyne into cis-form olefin
InactiveCN101704701AEfficient reductionHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventHydrogen
The invention discloses a method for reducing alkyne into cis-form olefin. The method comprises the following steps: in inert atmosphere, putting potassium hydroxide, a catalyst and alkyne in an organic solvent, and conducting reduction reaction on alkyne, and obtaining the cis-form alefin after the reaction. In the method, the catalyst is selected from at least one of Pd (OAc)2, PdCl2, Pd / C and Pdcl2 (PPh3). The organic solvent is N, N-dimethyl fomamide and / or at least one mixture in the following solvents: toluene, dioxane, dimethyl sulfoxide and water, and N,N-dimethyl fomamide is selectedpreferably. The alkyne is aliphatic alkyne or aromatic alkyne. The method adopts cheap and safe potassium hydroxide and DMF as the precursor of hydrogen, the catalyst is simple palladium compound which is cheap and easy to obtain, and the catalyst system shows the alkyne can be reduced into the cis-form olefin, with high efficiency and selectivity.
Owner:TSINGHUA UNIV
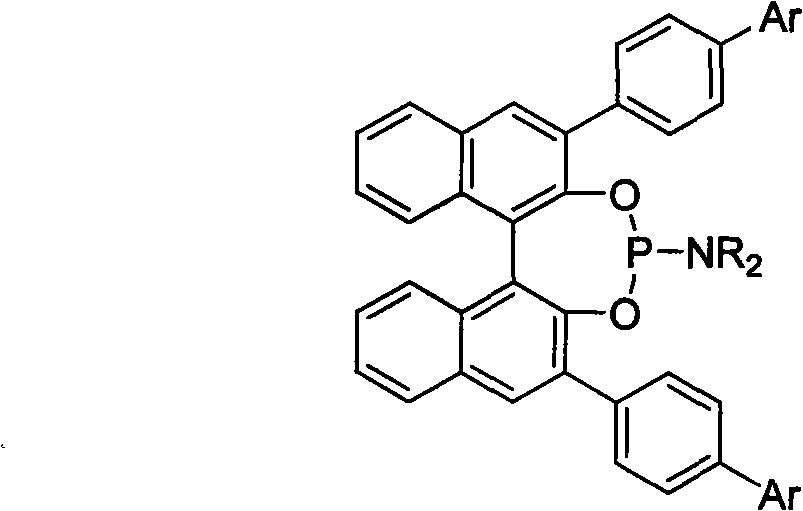
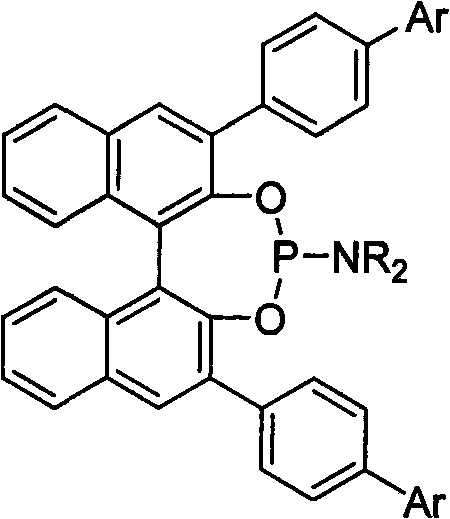
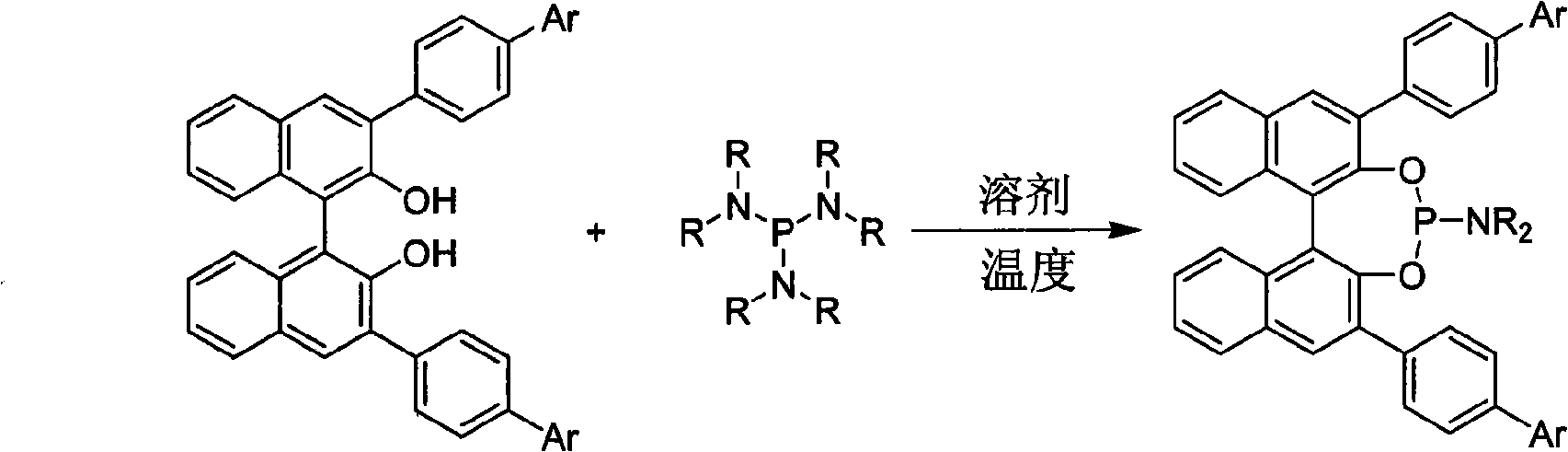
3, 3'-position biaryl group binaphthyl shaft chiral phosphoramidite ligand and preparation method thereof
InactiveCN101565436AGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventPhosphorus trichloride
The invention relates to a 3, 3'-position binaphthyl shaft chiral phosphoramidite ligand containing biaryl group and a preparation method thereof. The preparation method comprises the following steps of: leading 3, 3'-dual-biaryl group-2, 2'-dinaphthol to react with hexagon-alkyl group phosphoramidite in organic solvent at the temperature of 0-120 DEG C according to the mol rate of 1:1, or leading 3, 3'- dual-biaryl group-2, 2'-dinaphthol to react with phosphorus trichloride and dialkyl amine in steps, and after complete reaction, the object chiral phosphoramidite ligand can be obtained through washing, extraction and separation. After going through complexation with metallic copper salt, the chiral phosphoramidite ligand can be used for catalyzing the conjugate addition reaction of zinc alkyl with alpha, beta-unsaturated carbonyl compounds, and an additional product is prepared with high yield up to 78-98% and high enantioselectivity ee value up to 80-98%.
Owner:TIANJIN UNIV
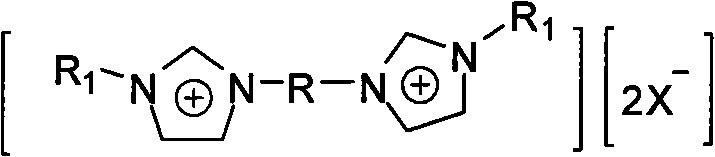
Gemini ionic liquid with fluorous biphasic property, and catalyst prepared by utilizing ionic liquid and application thereof
InactiveCN101781254ANo lossNot easy to loseOrganic-compounds/hydrides/coordination-complexes catalystsOrganic isomerisationIsomerizationIonic liquid
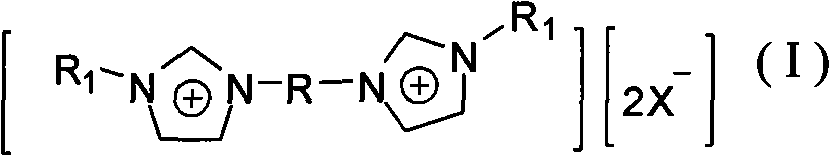
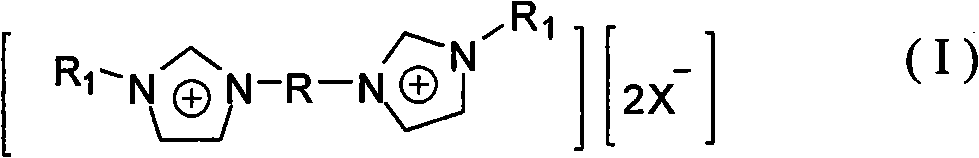
The invention discloses gemini ionic liquid with fluorous biphasic property. The molecular formula of the ionic liquid is [(R1N2C3H3)2R]2+.[2X-]. The structure of the ionic liquid is shown as the general formula (I) in the specification, wherein R1 represents hydrogen or C1-C8 alkyl; R represents ethylidene oxyl element, is selected from-(CH2CH2O)qCH2CH2- and is formed by polyethylene glycol 400-10000, and q is 6-250; and X represents HBF4, HPF6, HNO3 or halogenated hydrogen. A catalyst can be prepared immediately after adding palladium, rhodium or nickel catalyst into the gemini ionic liquid with fluorous biphasic property for coordination, and can be used in HECK reaction, C-C coupling reaction and isomerization reaction. The ionic liquid is not easy to volatilize, and the catalyst prepared by utilizing the ionic liquid is used in HECK reaction, C-C coupling reaction and isomerization reaction, has high catalytic efficiency and can be reused.
Owner:NANJING UNIV OF SCI & TECH
Alpha-menaphthyl substituted spiro bis(oxazoline) ligands, synthetic method and application thereof in synthesizing pyrazolidine derivatives
ActiveCN101560191AThe synthesis method is simpleMild conditionsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic cyclisationRegioselectivityChirality
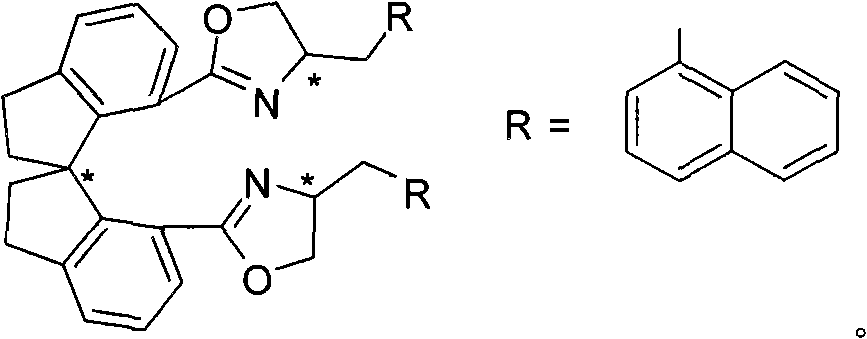
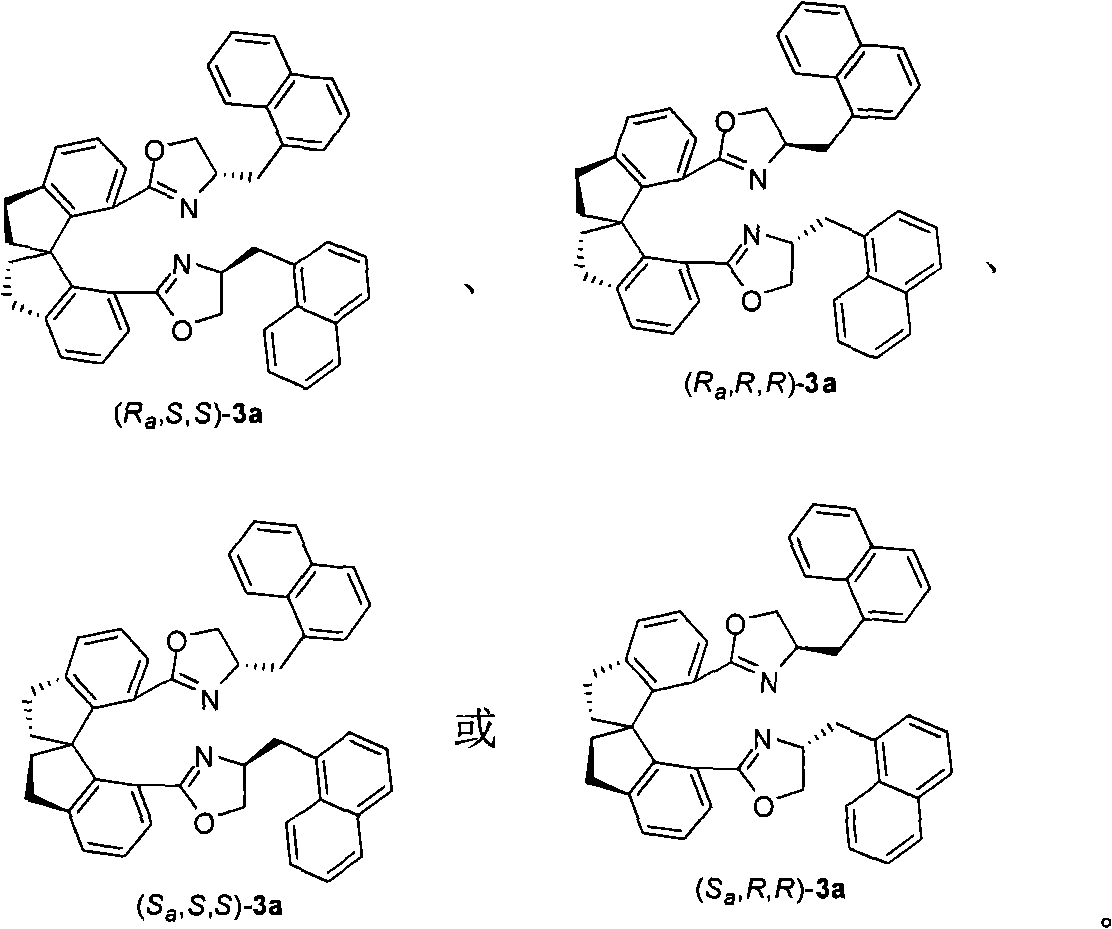
The invention provides spiro bis(oxazoline) ligands with a plurality of chiral centers, a preparation method and application. The ligands are provided with axial chirality of a spiro backbone and central chirality of an oxazoline ring. The ligands can be prepared by condensation of chiral spiro diacid and corresponding alkamine. The invention also provides application of the spiro bis(oxazoline) ligands in synthesizing pyrazolidine derivatives in high regioselectivity and high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Continuous polymerization process and products therefrom
InactiveUS6566549B1Polydispersity of increasesWeight increaseOrganic reductionOrganic detergent compounding agentsAcetic acidPolymer science
A continuous process for oligomers which do not contain, as polymerized units, carboxylic acid-containing monomers and their salts, including the steps of:(1) forming a reaction mixture, substantially free of carboxylic acid-containing monomers and their salts, containing:(i) 0.5 to 99.95% by weight of the reaction mixture of at least one ethylenically unsaturated monomer; and(ii) 0.05 to 25% by weight, based on the weight of the ethylenically unsaturated monomer, of at least one free-radical initiator; and(2) continuously passing the reaction mixture through a heated zone wherein the reaction mixture is maintained at a temperature of at least 150° C. and a pressure of at least 30 bars for from 0.1 seconds to 4 minutes to form terminally-unsaturated oligomers.In addition, processes for forming oligomers of vinyl acetate and oligomers of vinyl alcohol are disclosed. Mixtures of fully saturated and terminally unsaturated oligomers are also disclosed.
Owner:ROHM & HAAS CO
Method for preparing high dispersion supported hydrogenation catalyst
InactiveCN101549284AImprove catalytic performanceHigh catalytic activityOrganic compound preparationCatalyst activation/preparationAlloy catalystActive component
The present invention provides a method for preparing high dispersion supported hydrogenation catalyst. Alumina sol is used as a supporting material for preparing a NiB amorphous alloy catalyst with highly dispersed active component through a simple chemical reduction method. Excellent catalytic activity is represented in a reaction in which 4-nitrophenol hydrogenation is used as a probe. The activity is further higher than the NiB / r-Al2O3 catalyst which is prepared through a simple method. The catalyst has the advantages of high activity, simple preparing method, short preparing period, low cost, good reusability and excellent application prospect.
Owner:NANKAI UNIV +1
Preparation method of alpha, beta-unsaturated carboxylic acid compounds
ActiveCN105566021AReduce usageWide compatibilityCarboxylic acid nitrile preparationOrganic compound preparationPhosphineFunction group
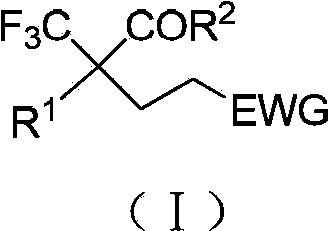
The invention provides a preparation method of alpha, beta-unsaturated carboxylic acid compounds. The method is characterized in that compounds represented by formula (I) react with formic acid in the presence of a nickel-containing catalyst, a phosphine ligand and an organic solvent to obtain the alpha, beta-unsaturated carboxylic acid compounds represented by formula (II), wherein R1 and R2 are respectively independently selected from H, C1-C30 alkyl groups, C1-C30 substituted alkyl groups, C1-C30 alkenyl groups, C1-C30 substituted alkenyl groups, C6-C30 aryl groups and C6-C30 substituted aryl groups. Compared with the prior art, the method adopting formic acid as a carboxylation reagent has the advantages of low price, safety, stability, low toxicity, high yield, simple operation, good economy, avoiding of use of precious metal catalysts and toxic gas carbon monoxide, meeting of requirements of environmentally friendly compounds, wide function group compatibility, high conversion rate and industrial synthesis values.
Owner:UNIV OF SCI & TECH OF CHINA
Process for preparing and olefinic hydrocarbon mixture
InactiveUS7148388B2Oxygen-containing compound preparationPreparation by oxo-reaction and reductionButeneHydrocarbon mixtures
In a process for preparing an olefinic hydrocarbon mixture comprising at least 5% by weight of mono-olefin oligomers of the empirical formula:CnH2n where n is greater than or equal to 6, a feedstock comprising n-butene and propylene in a molar ratio of about 1:0.01 to about 1:0.49 is contacted under oligomerization conditions with surface deactivated ZSM-23. The resultant mono-olefin oligomers comprise at least 20 percent by weight of olefins having at least 12 carbon atoms, wherein said olefins having at least 12 carbon atoms have an average of from about 0.8 to about 2.0 C1–C3 alkyl branches per carbon chain.
Owner:EXXONMOBIL CHEM PAT INC
Continuous polymerization process and products therefrom
InactiveUS20020082371A1Reduce the amount requiredHigh molecular weightOrganic reductionOrganic detergent compounding agentsAcetic acidPolymer science
A continuous process for oligomers which do not contain, as polymerized units, carboxylic acid-containing monomers and their salts, including the steps of: (1) forming a reaction mixture, substantially free of carboxylic acid-containing monomers and their salts, containing: (i) 0.5 to 99.95% by weight of the reaction mixture of at least one ethylenically unsaturated monomer; and (ii) 0.05 to 25% by weight, based on the weight of the ethylenically unsaturated monomer, of at least one free-radical initiator; and (2) continuously passing the reaction mixture through a heated zone wherein the reaction mixture is maintained at a temperature of at least 150° C. and a pressure of at least 30 bars for from 0.1 seconds to 4 minutes to form terminally-unsaturated oligomers. In addition, processes for forming oligomers of vinyl acetate and oligomers of vinyl alcohol are disclosed. Mixtures of fully saturated and terminally unsaturated oligomers are also disclosed.
Owner:GREENBLATT GARY DAVID +7
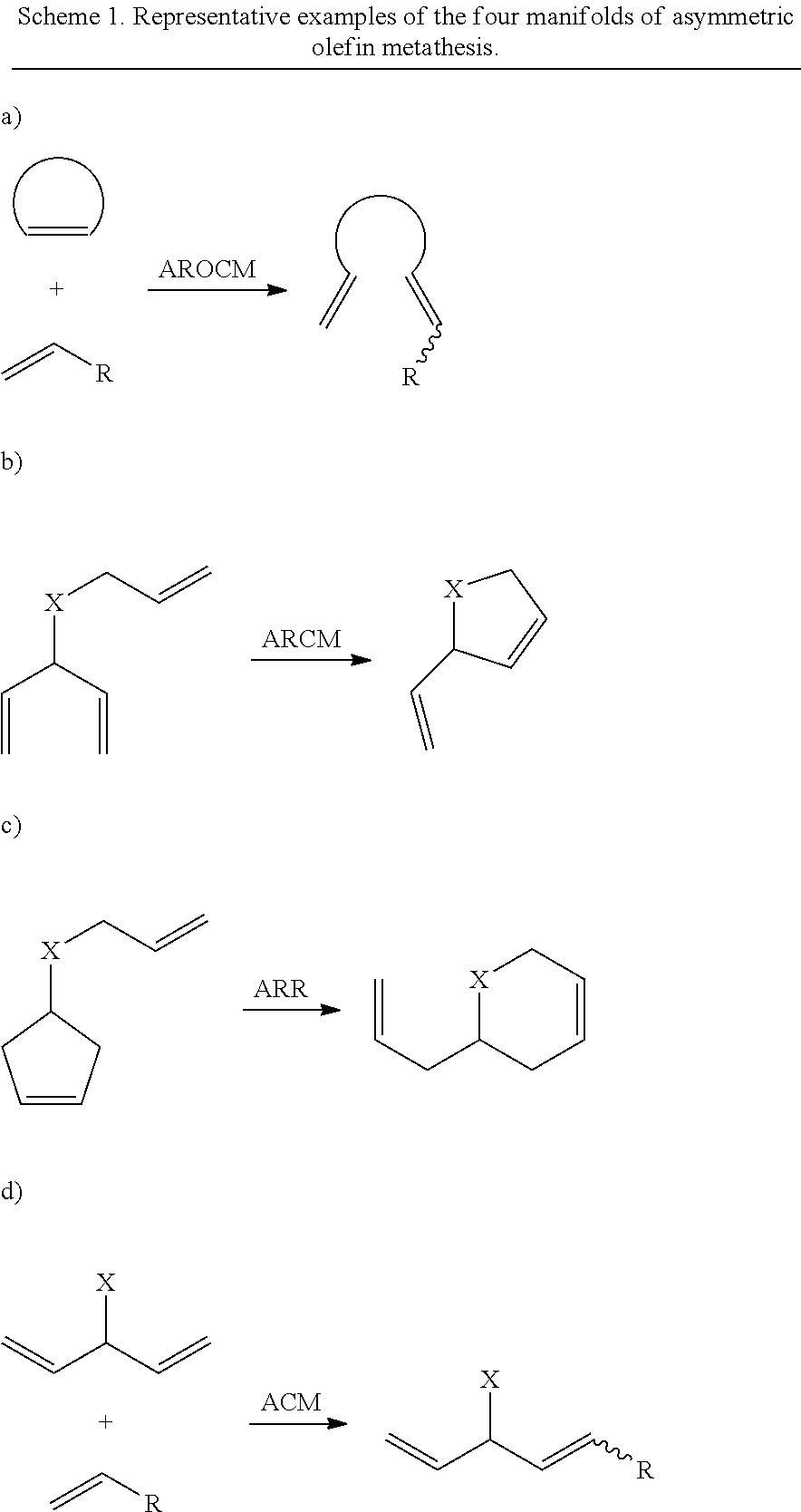
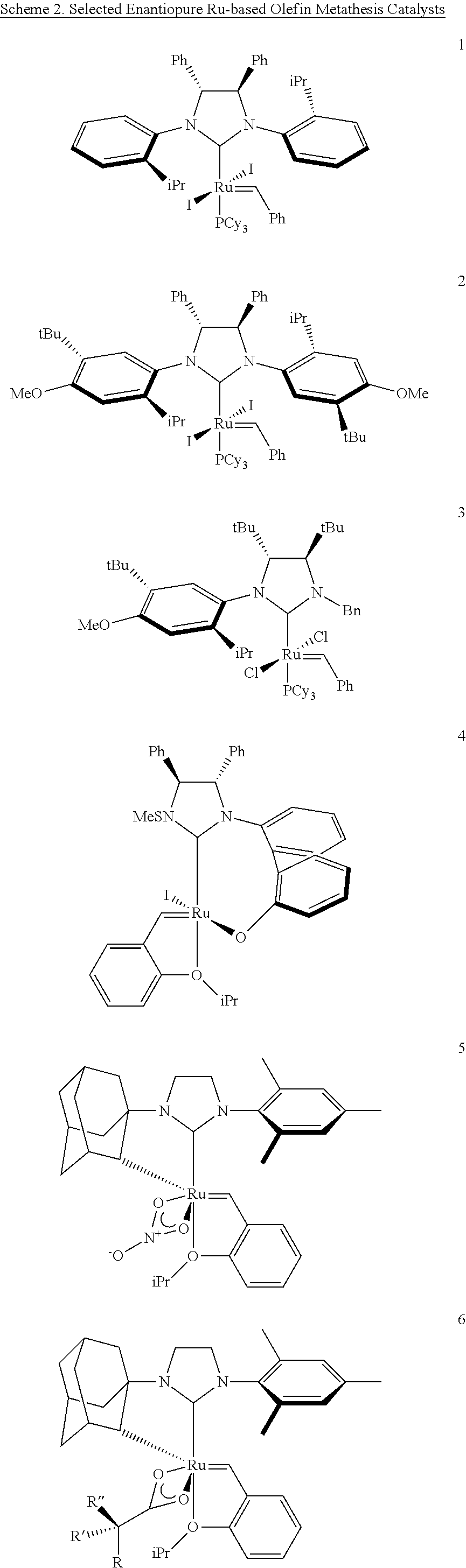
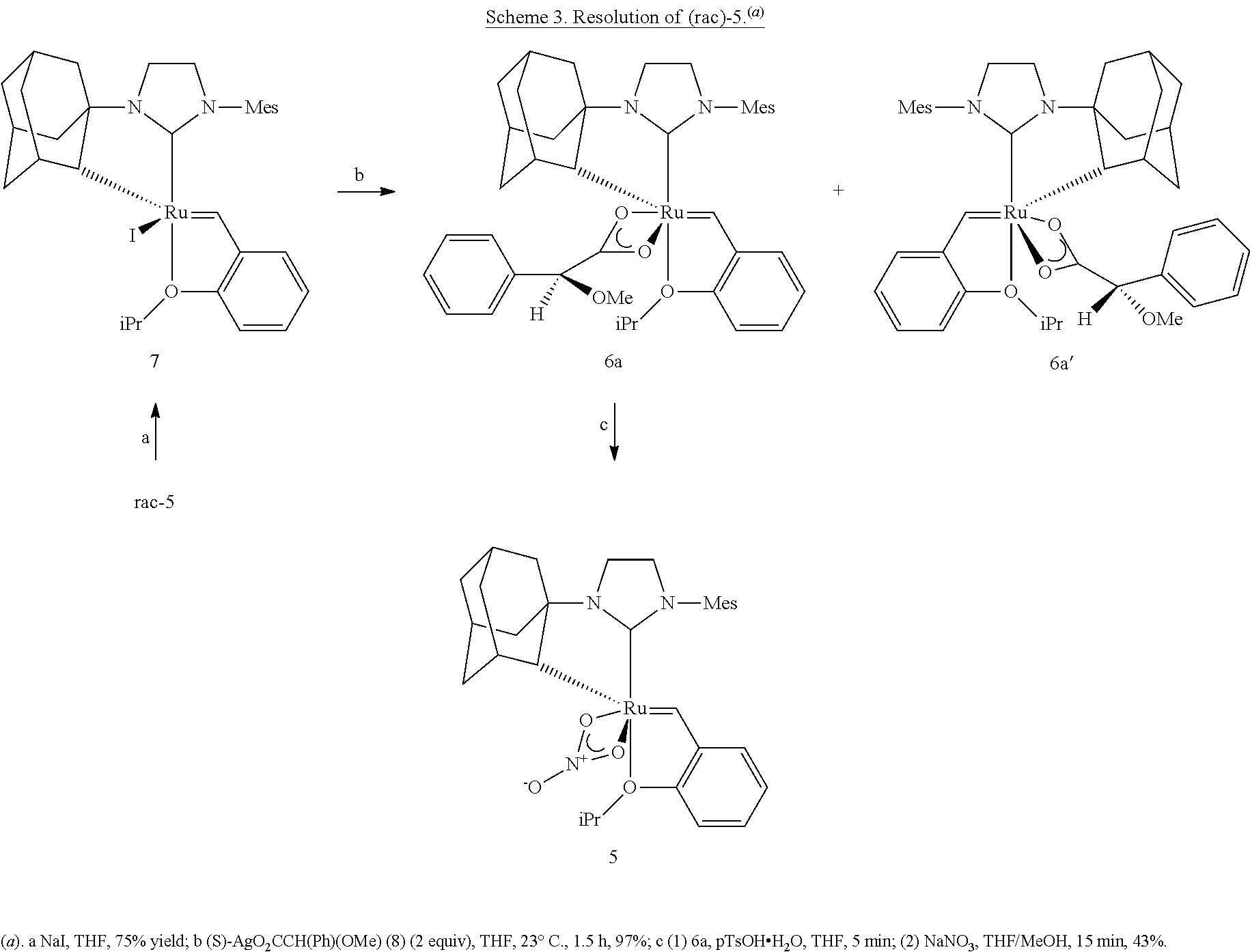
Selective olefin metathesis with cyclometalated ruthenium complexes
ActiveUS20160185684A1Quick breakdownLarge solubilityCarboxylic acid esters preparationHydroxy compound preparationRutheniumAlkene
This invention relates generally to C—H activated ruthenium olefin metathesis catalyst compounds which are stereogenic at the ruthenium center, to their preparation, and the use of such catalysts in the metathesis of olefins and olefin compounds. In particular, the invention relates to the use of C—H activated ruthenium olefin metathesis catalyst compounds in Z-selective olefin metathesis reactions, enantio-selective olefin metathesis reactions, and enantio-Z-selective olefin metathesis reactions. The invention has utility in the fields of catalysis, organic synthesis, polymer chemistry, and industrial and fine chemicals chemistry.
Owner:CALIFORNIA INST OF TECH
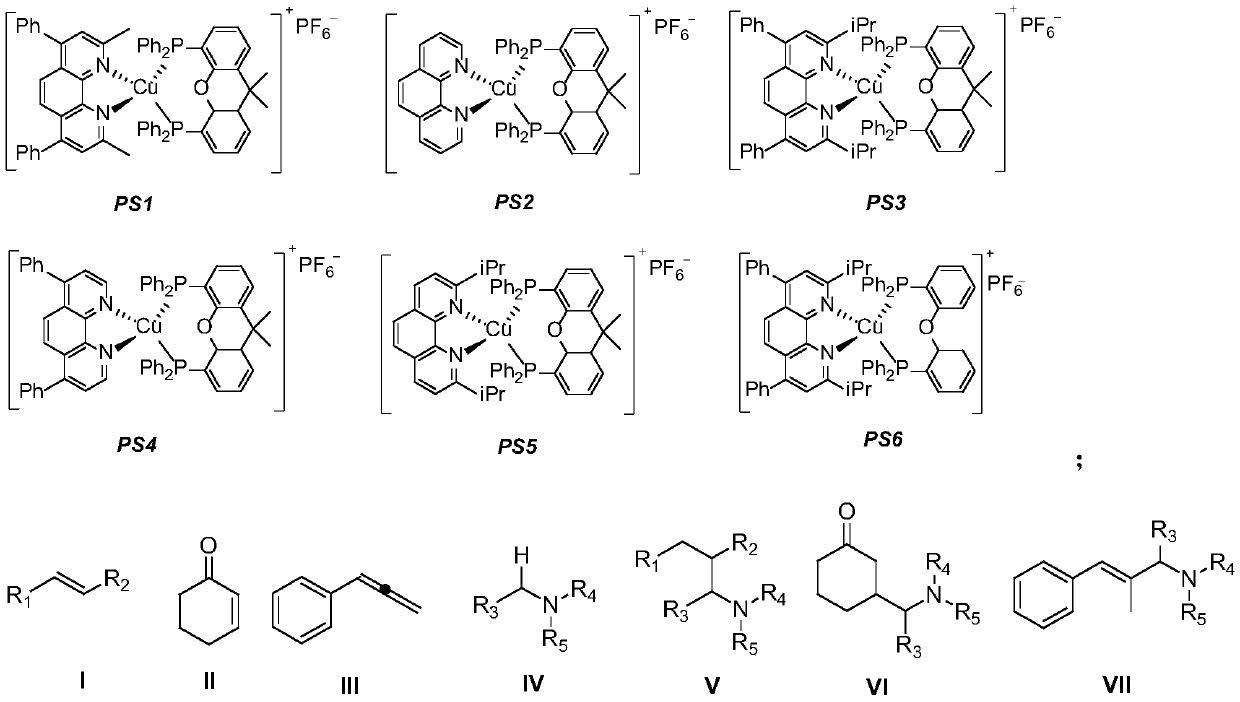
Synthesis method of photocatalytic tertiary amine compound
ActiveCN110790670AReduce consumptionUniversalAmino preparation from aminesOrganic compound preparationPtru catalystPhotosensitizer
The invention discloses a synthesis method of a photocatalytic tertiary amine compound. The method is specifically carried out according to the steps of: adding a photosensitizer and an alkaline substance into a Schlenk reaction tube, in a nitrogen atmosphere, dissolving olefin shown as formula I, formula II or formula III and tertiary amine shown as formula IV in an organic solvent to obtain a mixed solution, adding the mixed solution into the above reaction tube, carrying out stirring reaction for 6-14h at 25DEG C under the irradiation of a light source, and performing aftertreatment on theobtained reaction solution to obtain an amine compound shown as formula V, formula VI or formula VII respectively; wherein the mole ratio of the photosensitizer, the alkaline substance, the olefin shown as formula I, formula II or formula III and the tertiary amine shown as formula IV is 0.05:1-2:1:1.5-2.5. The method disclosed by the invention can be used for synthesis of tertiary amine that is difficult to prepare by existing method; a cheap and easily available copper-based catalyst is employed to replace a noble metal catalyst, and the toxicity is low; and the method has the advantages ofmild reaction conditions, saving of energy consumption, high yield, strong substrate universality, simple operation and the like.
Owner:ZHEJIANG UNIV OF TECH
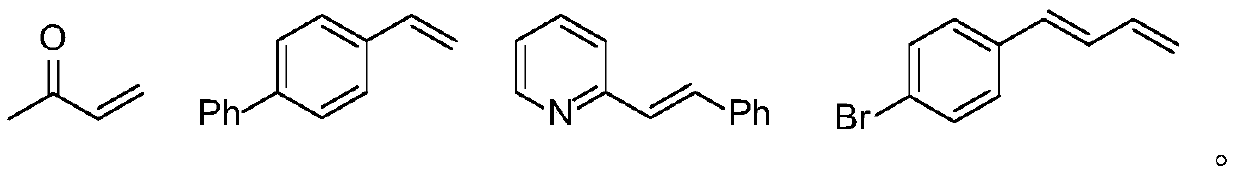
Method for synthesizing optically active trifluoromethyl compound by asymmetric conjugate addition reaction of organic boronic acid and alpha, beta-unsaturated ketone
ActiveCN110078605AHigh yieldHigh enantioselectivityOrganic compound preparationCarbonyl compound preparationTrifluoromethylationBoronic acid
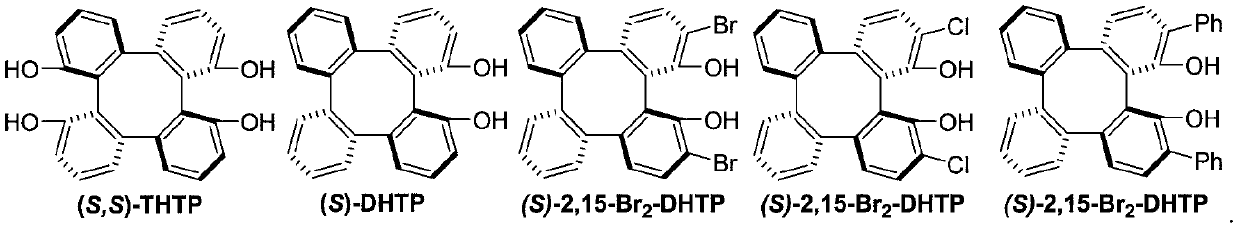
The invention discloses a method for synthesizing an optically active trifluoromethyl compound by asymmetric conjugate addition reaction of an organic boronic acid and alpha, beta-unsaturated ketone,and belongs to the technical field of asymmetric synthesis in the organic chemistry. A reaction equation is as shown in the specification. The method comprises the following specific steps: taking beta-CF3-alpha, beta-unsaturated ketone 1 and organic boric acid 2 as raw materials, and carrying out the asymmetric conjugate addition reaction to obtain the trifluoromethyl compound in the presence ofchiral tetrabenzocyclooctatetraenic or chiral binaphthol catalysts, as well as molecular sieves and magnesium tert-butoxide additives, wherein R<1> is selected from phenyl, substituted phenyl, 2-naphthyl, 1-naphthyl, 2-thienyl, 3-thienyl and cyclohexyl, and R<2> is selected from styryl, 2-furanyl and 2-benzofuranyl. The method has the advantages that reaction raw materials are easy to obtain, reaction conditions are mild, post-treatment is simple, the catalyst can be recycled and reused, the product yield and the enantioselectivity are good to excellent, and the product contains a trifluoromethyl chiral center.
Owner:HENAN NORMAL UNIV
Preparation method of trifluoromethyl carbonyl compound
The invention discloses a preparation method of a trifluoromethyl carbonyl compound. The preparation method is characterized in that organic micromolecule phosphine is adopted to catalyze a fluorine-containing material and alpha, beta-unsaturated compounds to generate michael addition reaction, wherein a beta defluorination phenomenon is effectively avoided in the reaction process; moreover, compared with the existing synthetic method, the materials and the catalyst are cheap and easily available, reaction conditions are gentle and easy to control, the usage amount of the catalyst is less, post-treatment is simple, yield is high, application prospect is wide; meanwhile, carboxylic compounds containing alpha- trifluoromethyl also can be directly prepared after further hydrolysis of the alpha- trifluoromethyl ether compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Methods, systems and catalysts for use in aromatic alkylation reactions
InactiveUS20050043573A1Organic-compounds/hydrides/coordination-complexes catalystsHydrocarbon purification/separationAlkyl transferSystems approaches
The present invention provides a system, method and catalyst for catalyzing alkylation reactions. The method includes catalyzing an alkylation reaction using a base treated, sulfonated, halogenated, and optionally acid regenerated thermally stable catalyst.
Owner:RAMPRASAD DORAI +1
Compositions and methods of promoting organic photocatalysis
ActiveUS20180237550A1Improve responseOrganic-compounds/hydrides/coordination-complexes catalystsOrganic halogenationAtom-transfer radical-polymerizationQuenching
The invention provides novel compounds and methods that are useful in promoting reactions that proceed through an oxidative quenching pathway. In certain embodiments, the reactions comprise atom transfer radical polymerization.
Owner:COLORADO STATE UNIVERSITY +1
Preparation method of substituted cis-olefin
ActiveCN104926577AHigh selectivityEasy to operateOrganic compound preparationHydrocarbon by hydrogenationHydrogen pressureAlkyne
The invention belongs to the technical field of medicine and natural compound chemical intermediates and related chemistry, and relates to a preparation method of substituted cis-olefin. The method includes using alkyne and derivatives thereof as raw materials, a nanoporous gold catalyst as a catalyst, hydrogen gas as a hydrogen source and an organic alkali as a solvent, and performing selective hydrogenation to prepare cis-olefin, wherein hydrogen pressure is 0.1-20.0MPa; and molar concentration of alkyne and derivatives thereof in the solvent is 0.01-2mmol / mL. The catalyst is a nanoporous gold catalyst, the porous frame size is 5-50nm, and the molar ratio of alkyne and the derivatives thereof to the catalyst is 1:0.01-1:0.1. The method has the advantages of high product selectivity, and simple operation and post-treatment; and the catalyst is good in reproducibility, the catalytic effect is not significantly lowered after the catalyst is repeatedly used, which provides the possibility for industrialization.
Owner:DALIAN UNIV OF TECH
Novel biaryl-structured chiral N-methyl pyridoxal catalyst and synthesis and application thereof
ActiveCN108947894AImprove biological activitySynthesis shortcutGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsDiamino acidHydrogen
The invention relates to a novel biaryl-structured chiral N-methyl pyridoxal catalyst and synthesis and application thereof. The general structural formula is as shown in the specification, wherein R1is hydrogen or C1-C24 alkyl, and R2 and R3 are independently hydrogen or C1-C24 alkyl. In comparison with the prior art, the prepared pyridoxal catalyst can be used in a bionic Mannich reaction process, and fast and effective synthesis of a chiral alpha-beta-diamino acid derivative is realized.
Owner:SHANGHAI NORMAL UNIVERSITY
Complexes
ActiveUS20170120231A1Stably formReduce congestionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHeteroatomCoupling reaction
A palladium(II) complex of formula (1) or a palladium(II) complex of formula (3).Also, processes for the preparation of the complexes, and their use in carbon-carbon and carbon-heteroatom coupling reactions.
Owner:JOHNSON MATTHEY PLC
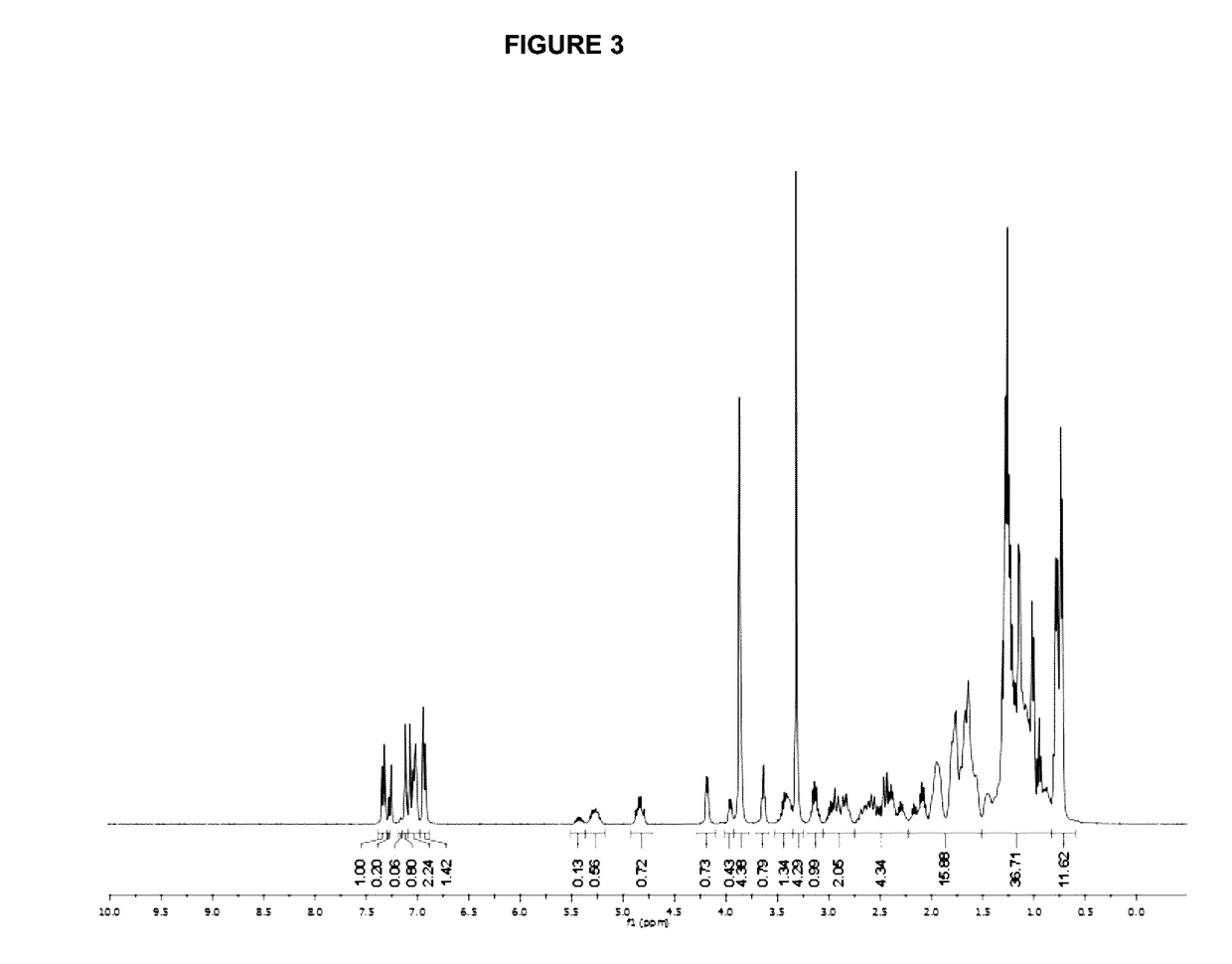
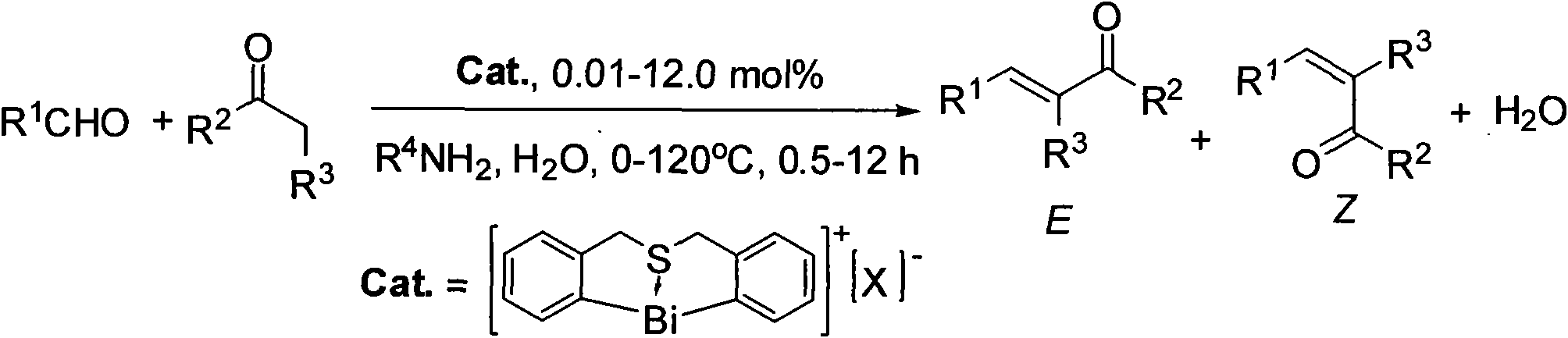
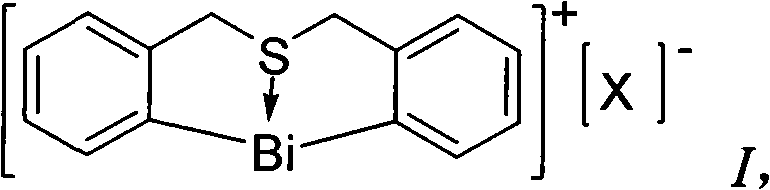
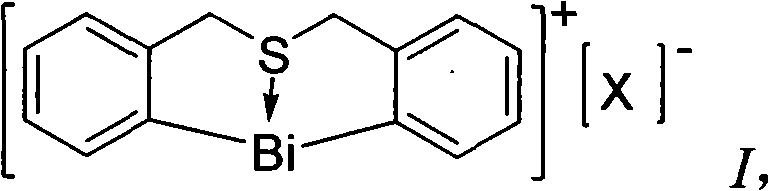
Method for synthesizing (E)-Alpha, Beta-unsaturated carbonyl compounds
InactiveCN101565341AWide variety of sourcesLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsSulfur
The invention provides a method for synthesizing (E)-Alpha, Beta-unsaturated carbonyl compounds, which takes aldehyde and ketone as raw materials, takes organic bismuth ion complexes containing bridged sulfur atom ligand as a main catalyst, takes aliphatic amine as an auxiliary catalyst, carries out a catalytic reaction in a protic solvent, thus preparing the (E)-Alpha, Beta-unsaturated carbonyl compounds. The synthesis method inaugurates a new low-cost and green approach for preparing the (E)-Alpha, Beta-unsaturated carbonyl compounds and has the advantages that the raw materials have wide source, the selectivity and the yield of the target outcome approach to 100%, the catalyst can be used repeatedly, the reaction condition is simple, the reaction is easy for operation, the cost is low and the preparation process is green and environment-friendly.
Owner:HUNAN UNIV
Method of forming polysaccharide structures
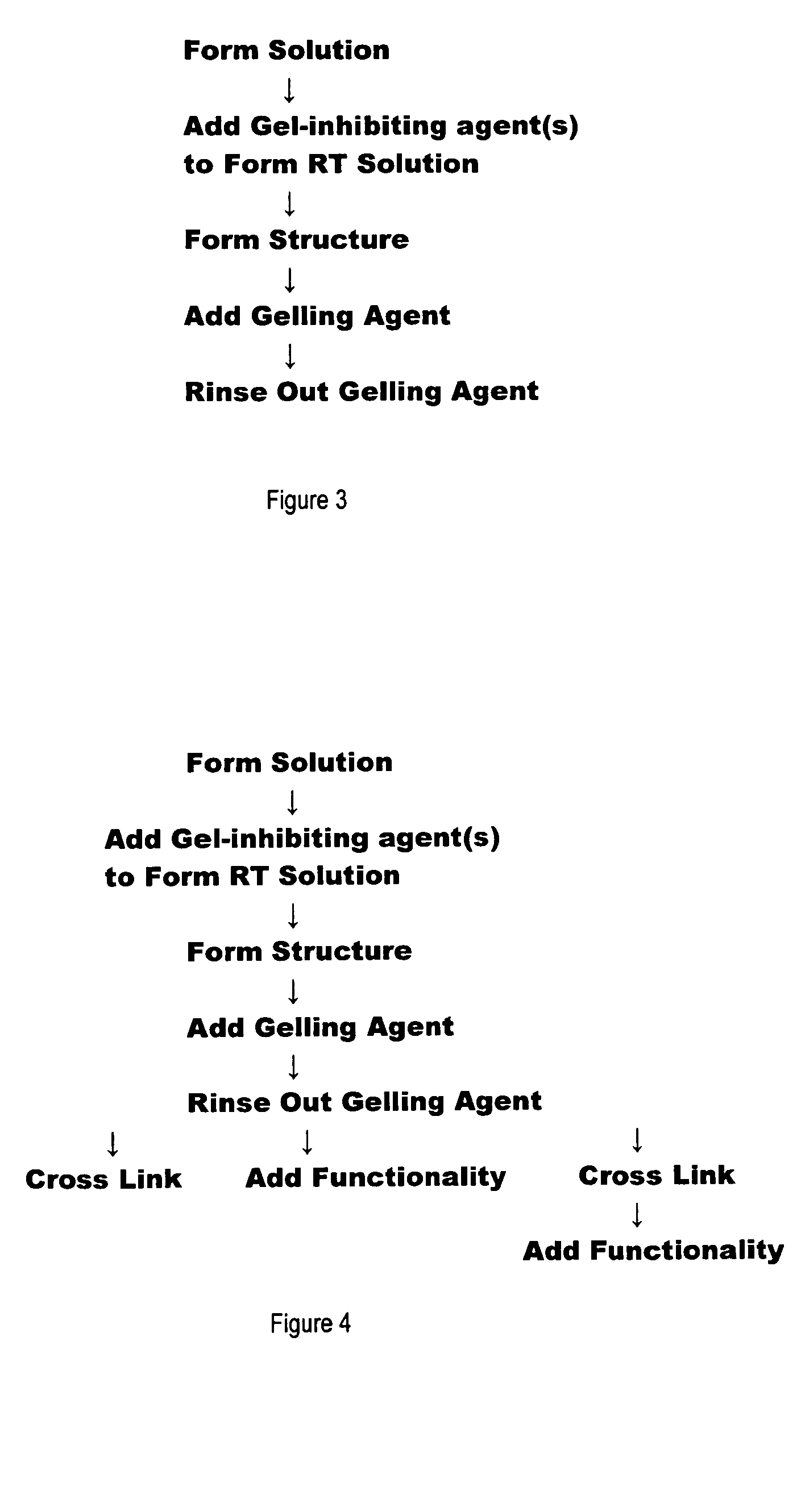
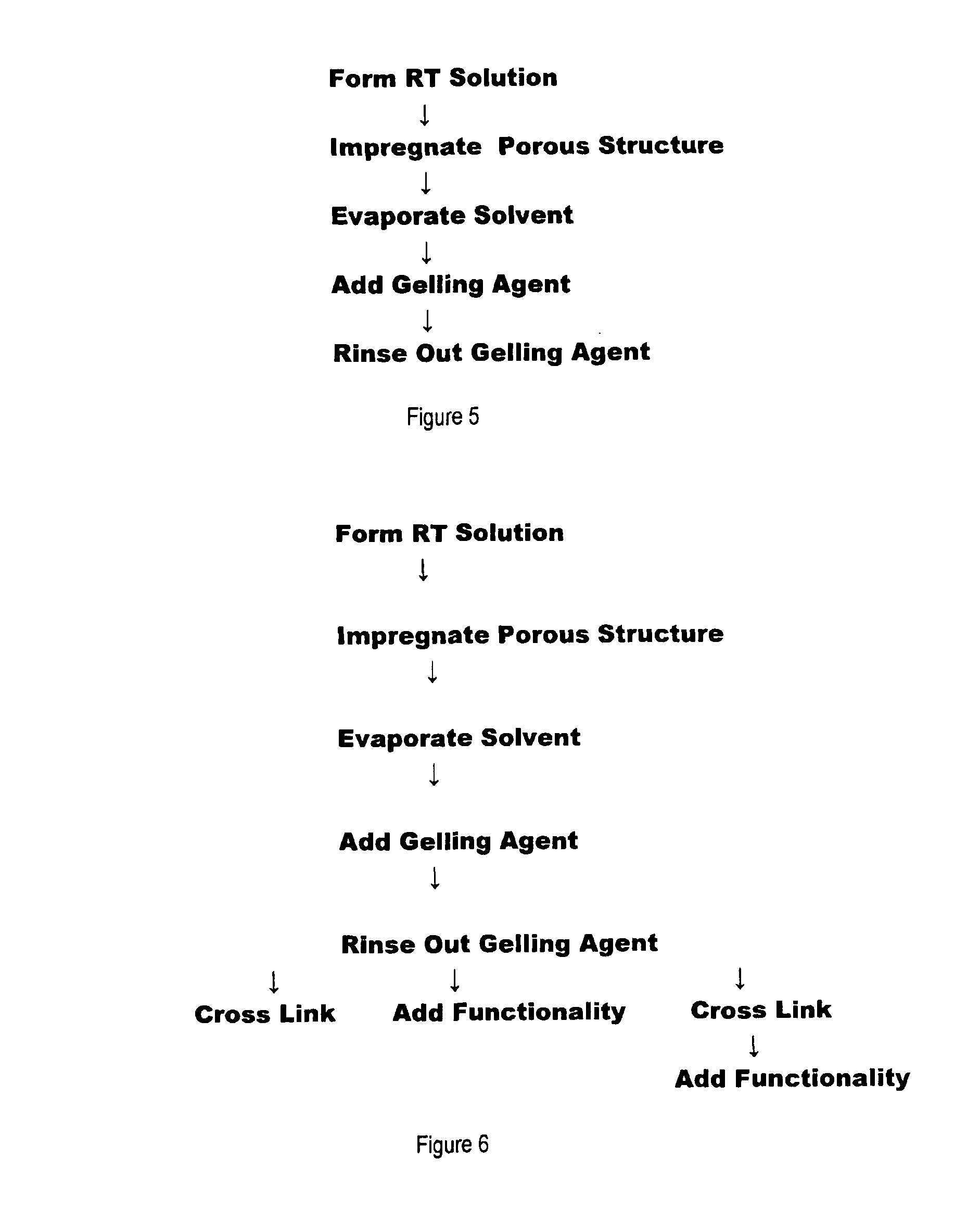
A process for forming polysaccharide structures such as beads, gel films and porous coatings on porous substrates by forming a room temperature gel-inhibited solution of a polysaccharide, one or more gel-inhibiting agent(s) and a solvent such as water, heating the mixture until all the components are dissolved, cooling the mixture as a solution to about room temperature, forming a three dimensional structure with the solution and adding the structure to a gelling agent to form a polysaccharide gel. Optionally, the solution can be added to a porous structure such as a non-woven fabric or a porous membrane and the solution is allowed to dry before being subjected to the gelling agent. Porous structures having a polysaccharide coating and being capable of convective flow through the pores of the structure and diffusive flow through the coating can be formed.
Owner:MILLIPORE CORP
Complexes
ActiveUS9777030B2Reduce congestionHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCouplingHeteroatom
A palladium(II) complex of formula (1) or a palladium(II) complex of formula (3).Also, processes for the preparation of the complexes, and their use in carbon-carbon and carbon-heteroatom coupling reactions.
Owner:JOHNSON MATTHEY PLC
Catalyst for Michael addition reaction and preparation method of nitro fatty aldehyde
ActiveCN102441431ADiastereomer goodGood choiceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenTrimethylsilyl
The invention provides a catalyst for a Michael addition reaction, which is characterized by comprising a compound shown by the formula I and a compound shown by the formula II, wherein R represents for dialkyl amino or naphthene amino; R1 and R2 respectively represent for hydrogen, bromine, iodine, phenyl, 2, 4, 6-trimethylphenyl, trimethylsilyl or triphenysilyl. The catalyst provided by the invention can be used for catalyzing fatty aldehyde and nitroolefin to perform an asymmetrical Michael addition reaction to obtain a product with high yield, high diastereomer selectivity and high enantiomer selectivity. The invention also provides a preparation method of nitro fatty aldehyde.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
A method for one-step synthesis of carboxylic acids with two extended carbon chains from olefins
ActiveCN111718228AAvoid interconversionEasy to operateOrganic compound preparationCarboxylic acid esters preparationDistillationCarboxylic acid
The invention relates to a method for one-step synthesis of carboxylic acid with two extended carbon chains from olefin, which comprises the following steps: under the protection of inert gas, sequentially adding an olefin substrate, a photocatalyst, a hydrogen atom transfer reagent, alpha-haloacetic acid, a reducing agent, a solvent and protonic acid into a reactor, and reacting at normal temperature under the irradiation of 25W blue light to obtain a reaction product; diluting, alkalizing, washing, acidifying and extracting the reaction product to obtain an organic phase; and finally, carrying out reduced pressure distillation and column chromatography on the organic phase to obtain a carboxylic acid product with two extended carbon chains; or carrying out reduced pressure distillation and column chromatography on the reaction product to obtain a carboxylic acid product with two extended carbon chains. The invention is simple to operate, direct synthesis conditions are mild, mutual conversion among various functional groups in the traditional carboxylic acid compound synthesis process is avoided, and the atom and step economy of the reaction is improved. Meanwhile, the method disclosed by the invention can also be applied to the simplified synthesis of the medicines cinacarbazide and tirofiban.
Owner:LANZHOU UNIVERSITY
Novel catalyst for aldol condensation reactions
InactiveCN101868440ALow costReduce usageOrganic compound preparationCarbonyl compound preparationStrong acidsKetone
The present invention presents catalytic systems for aldol condensation reactions, comprising the step of reacting at least one aldehyde or one ketone starting material in the presence of an inorganic ammonium salt, or an aqueous or organic solution prepared from such salt. The efficiency of the catalytic system of the present invention is comparable to those of the classical strong acid and strong base catalysts.
Owner:芭芭拉・诺齐埃尔
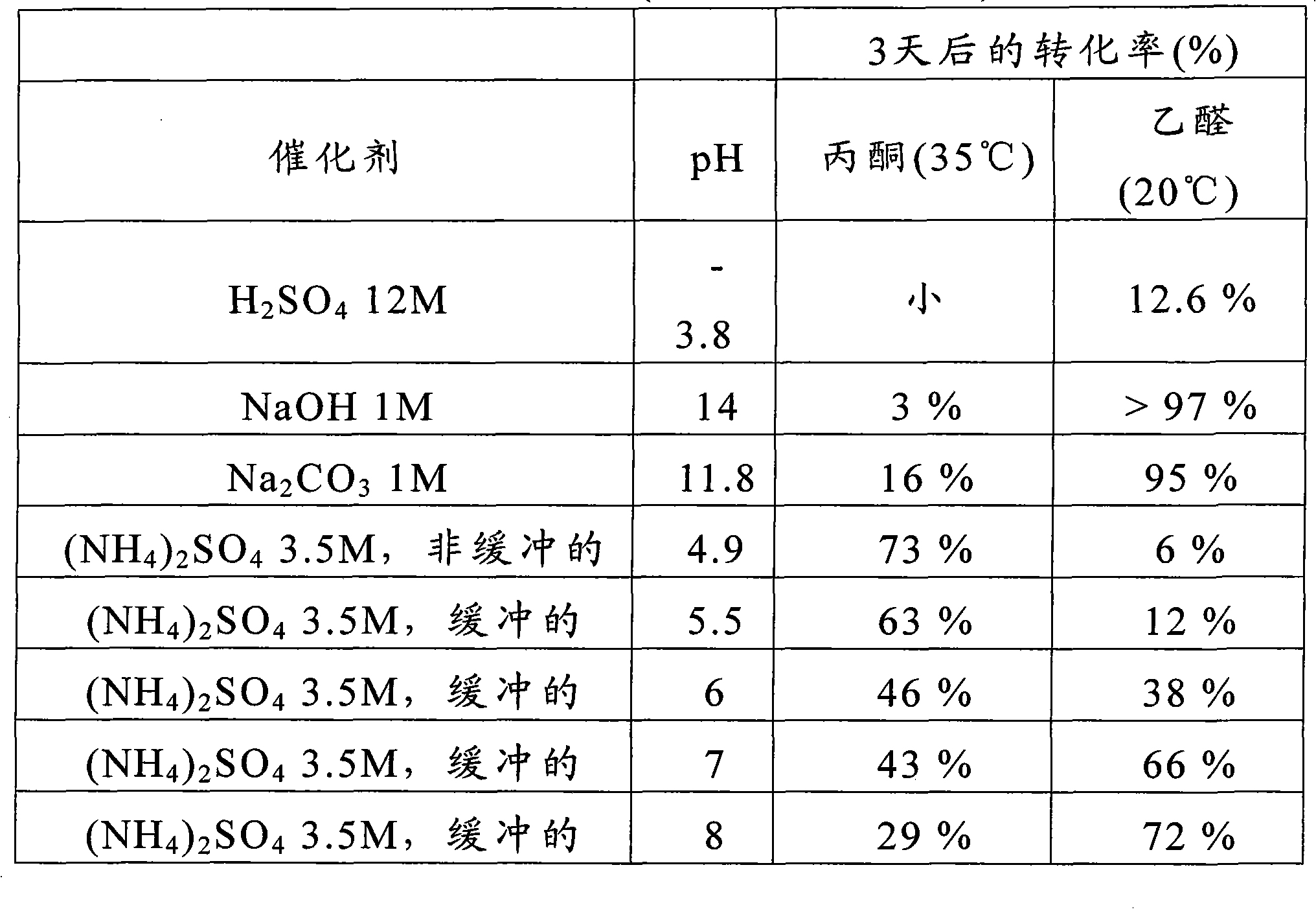
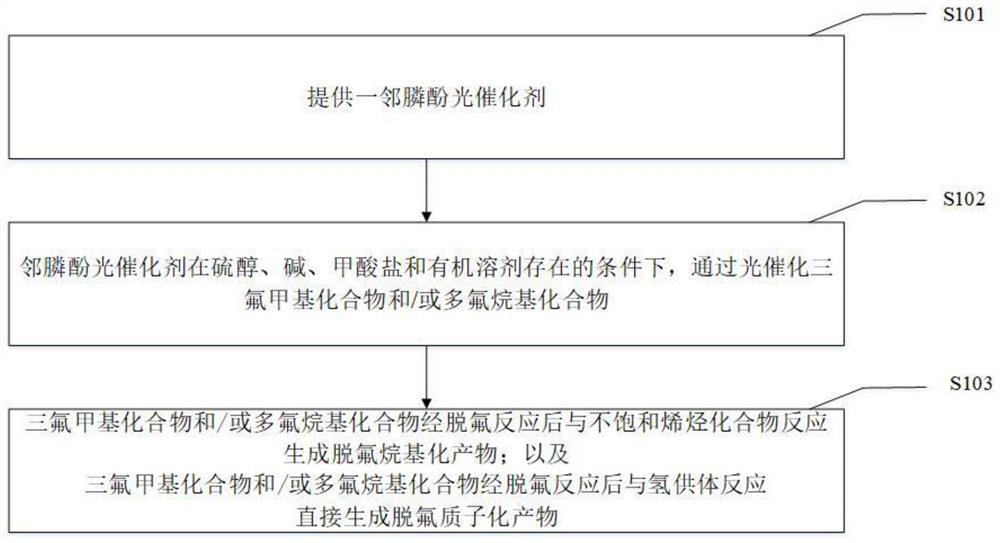
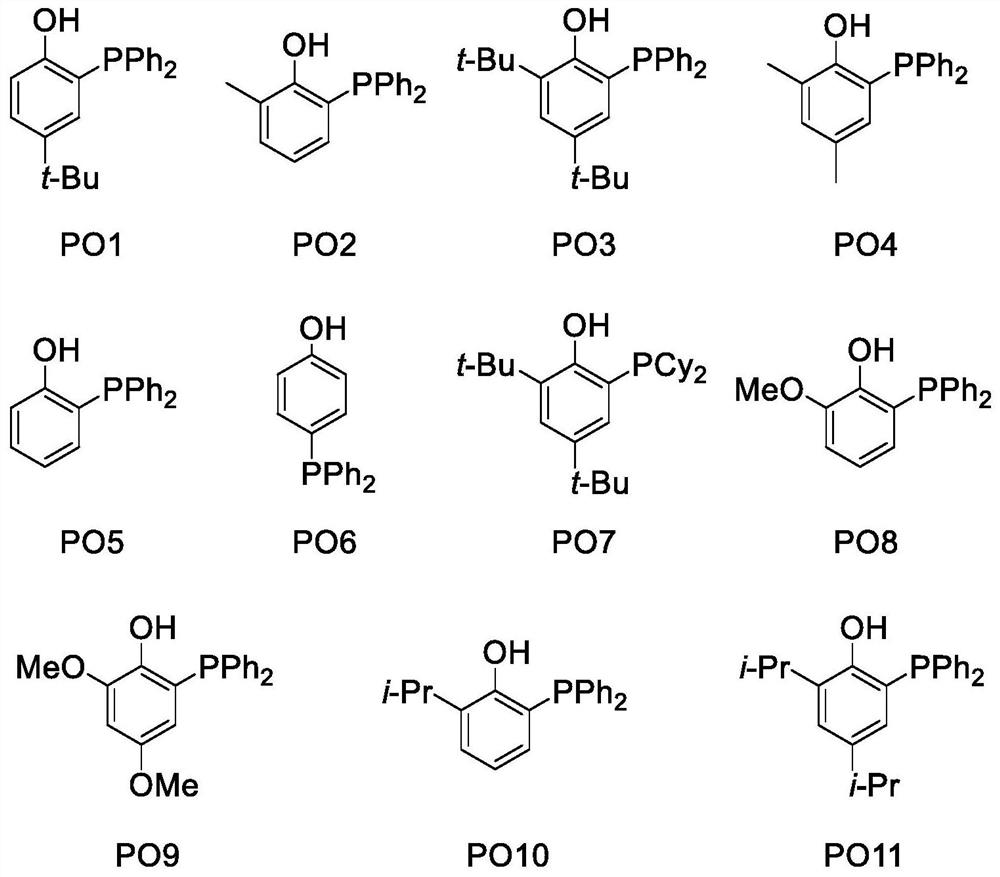
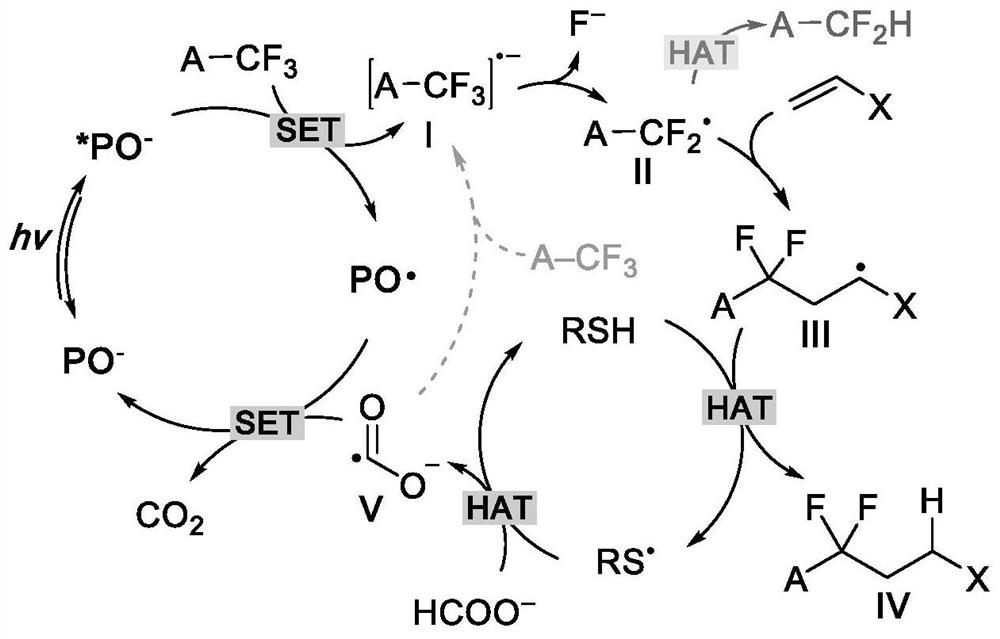
Method for defluorination alkylation and defluorination protonation reaction by using o-phosphinophenol photocatalyst
PendingCN113845436AStrong reductionEasy to crossGroup 4/14 element organic compoundsCarbamic acid derivatives preparationTrifluoromethylationOrganic solvent
The invention provides a method for defluorination alkylation and defluorination protonation reaction by using an o-phosphinophenol photocatalyst. The method comprises the following steps: providing an o-phosphinophenol photocatalyst, and carrying out photocatalysis on a trifluoromethyl compound (1a) R-CF3 and / or a polyfluoroalkyl compound (1b) R-CF2CF3 in the presence of mercaptan, an alkali, formate and an organic solvent; and reacting with an unsaturated olefin compound (2) to generate a defluorination alkylation product after the defluorination reaction of the trifluoromethyl compound and / or polyfluoroalkyl compound, and reacting with a hydrogen donor to directly generate a defluorination protonation product after defluorination reaction of the trifluoromethyl compound and / or polyfluoroalkyl compound.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com