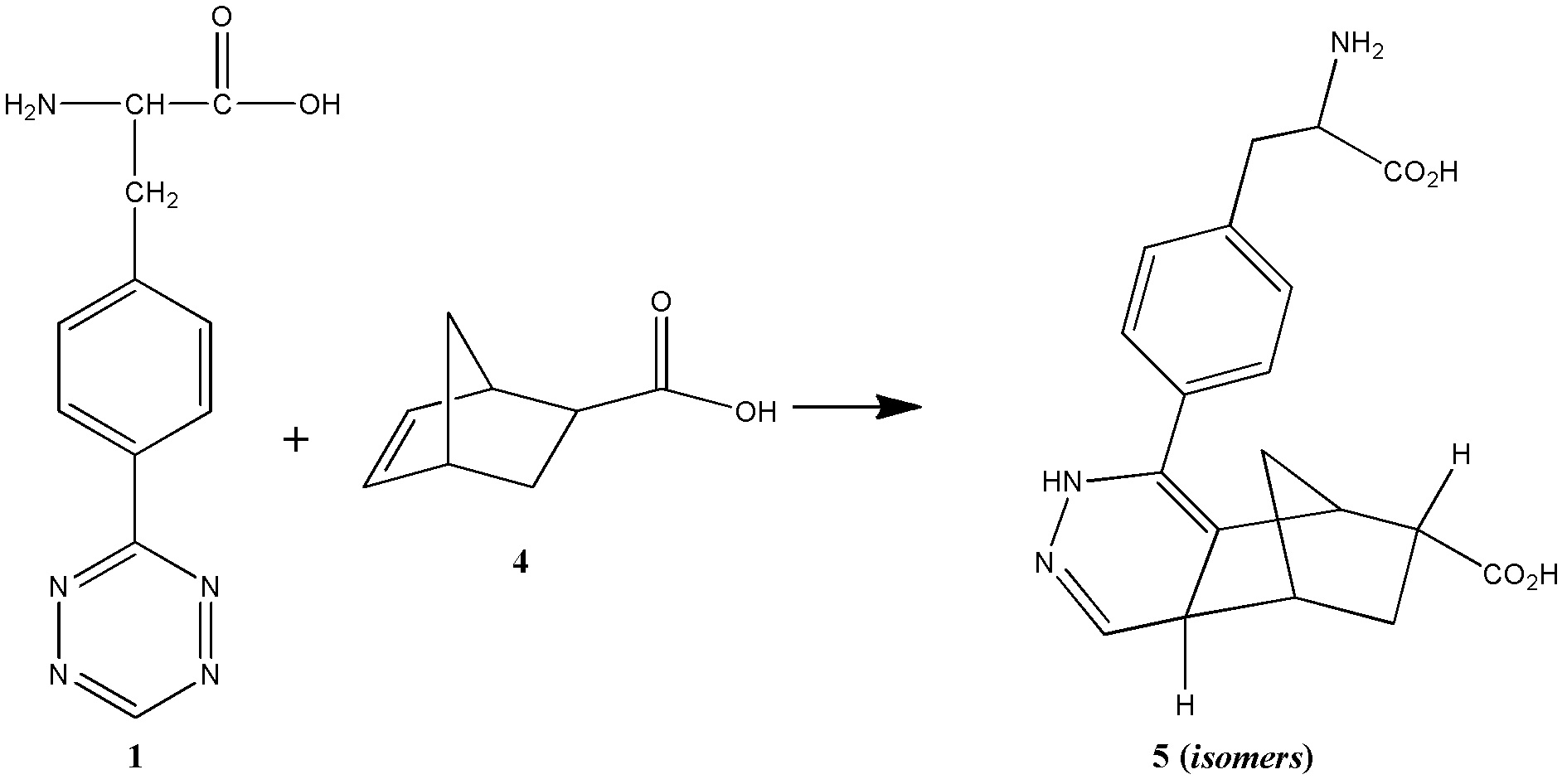
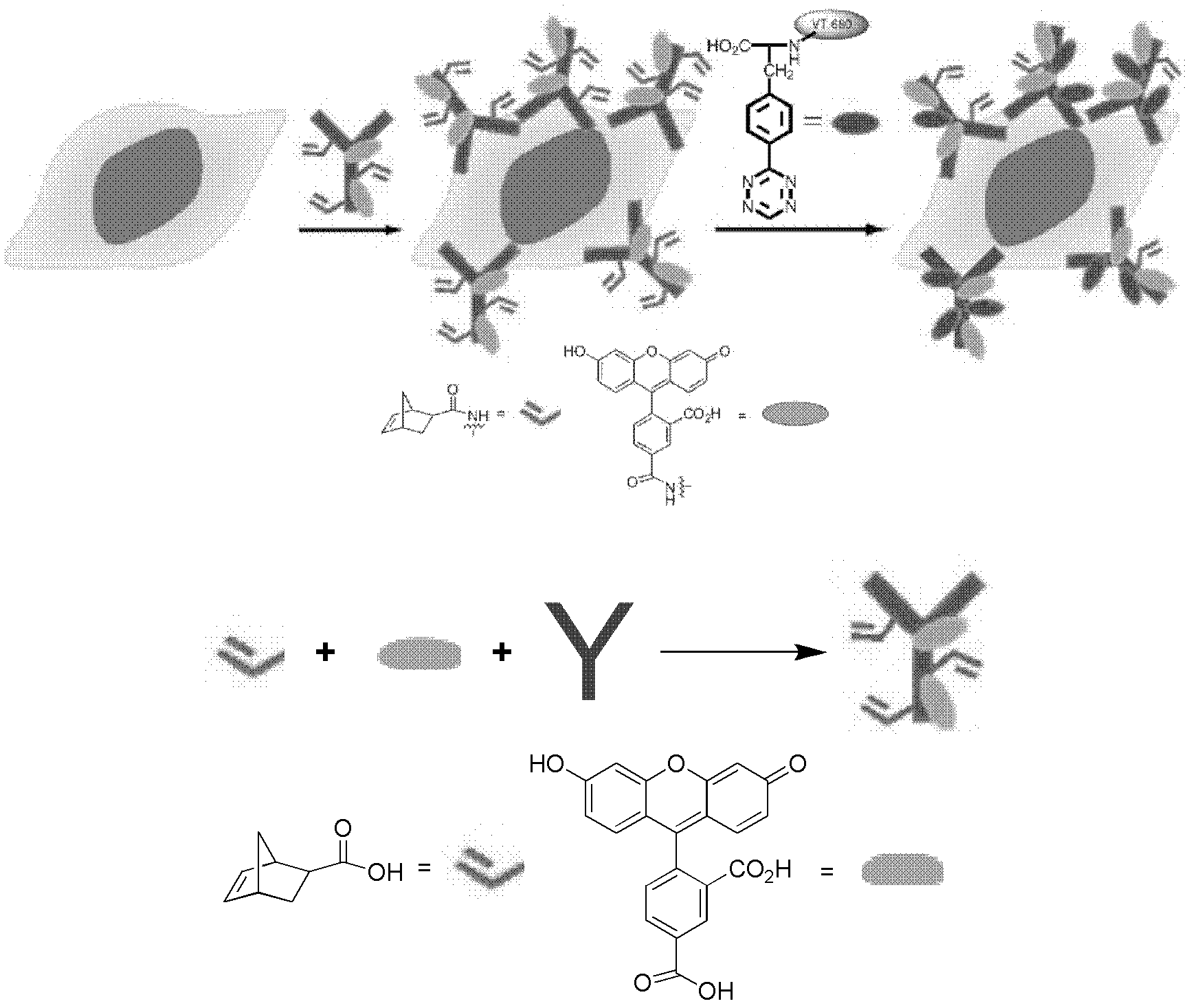
Novel compound L-4-terazine-phenylalanine, preparation method and application thereof
A technology of phenylalanine and L-4-, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as poor stability, and achieve the effects of easy preparation, high yield, and simplified steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
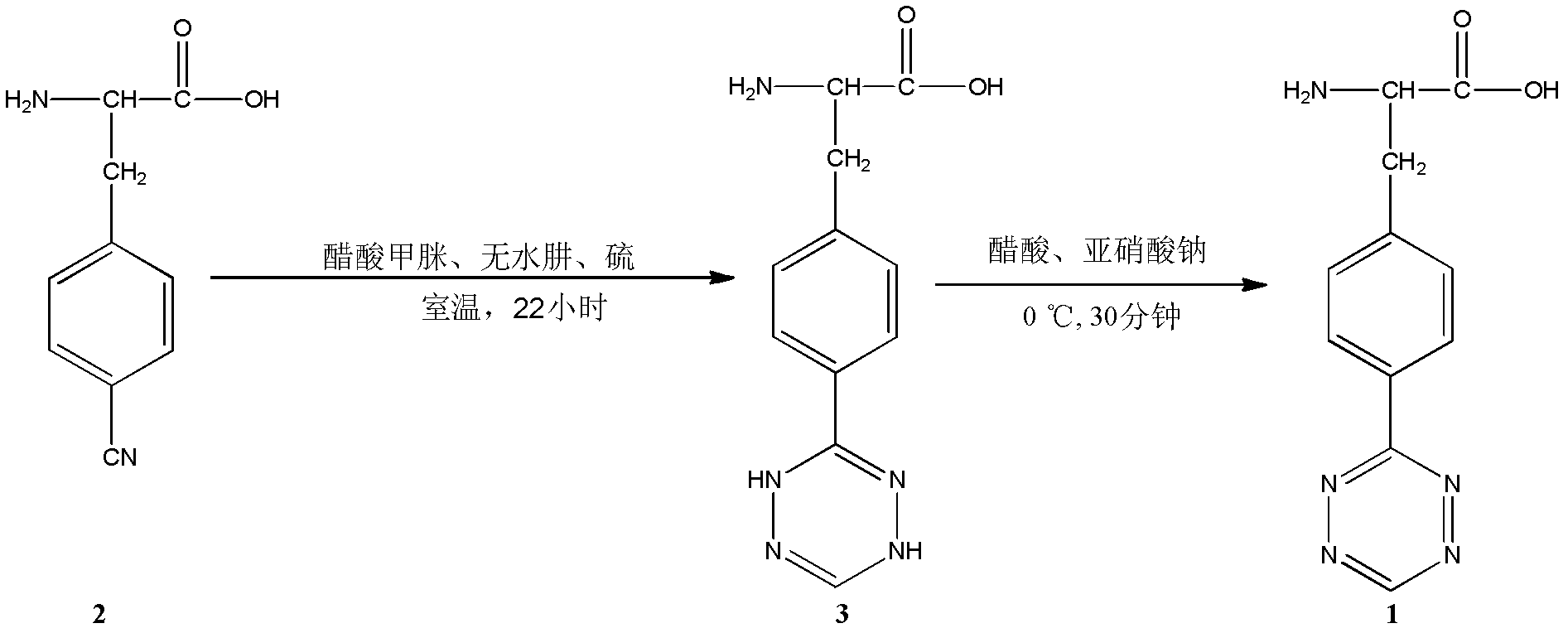
[0035] Weigh L-4-cyano-phenylalanine (2.5mmol, 0.475g), formamidine acetate (10mmol, 1.040g), sulfur (2.5mmol, 0.080g) and mix well, add anhydrous hydrazine (100mmol, 3.14 mL), after the addition of anhydrous hydrazine, gas was released and the reactant gradually turned into a yellow viscous substance. After stirring and reacting at room temperature for 22 hours, 15 mL of acetic acid was added to fully dissolve the yellow viscous substance, and the yellow solution was obtained by suction filtration. Weigh sodium nitrite (12.5mmol, 0.863g) and dissolve it in 1.5mL of water, and slowly add it into the above yellow solution under the condition of ice-water bath and stir thoroughly, that is, bubbles are formed, the solution turns purple, and react for 30min. Rotary evaporation removed as much acetic acid as possible to obtain a viscous oil, which was filtered with suction to obtain a purple solid, which was washed with dichloromethane and acetonitrile to obtain 0.3981 g of 4-tetraz...
Embodiment 2
[0037] Weigh L-4-cyano-phenylalanine (2.5mmol, 0.475g), formamidine acetate (5mmol, 0.520g), sulfur (2.5mmol, 0.080g) and mix well, add anhydrous hydrazine (25mmol, 0.785 mL), after the addition of anhydrous hydrazine, gas was released and the reactant gradually turned into a yellow viscous substance. After stirring and reacting at room temperature for 24 hours, 15 mL of acetic acid was added to fully dissolve the yellow viscous substance, and the yellow solution was obtained by suction filtration. Weigh sodium nitrite (10mmol, 0.69g) and dissolve it in 1.5mL of water, and slowly add it into the above yellow solution under the condition of ice-water bath and stir thoroughly, that is, bubbles are formed, the solution turns purple, and react for 60min. Rotary evaporation removed as much acetic acid as possible to obtain a viscous oil, which was filtered with suction to obtain a purple solid, washed with dichloromethane and acetonitrile to obtain 0.3652 g of 4-tetrazine-L-phenylal...
Embodiment 3
[0039]Weigh L-4-cyano-phenylalanine (2.5mmol, 0.475g), formamidine acetate (12.5mmol, 1.300g), sulfur (7.5mmol, 0.240g) and mix well, add anhydrous hydrazine (125mmol, 3.925mL), after the addition of anhydrous hydrazine, gas was released and the reactant gradually turned into a yellow viscous substance. After stirring and reacting at room temperature for 16 hours, 15mL of acetic acid was added to fully dissolve the yellow viscous substance, and the yellow solution was obtained by suction filtration. Weigh sodium nitrite (15mmol, 1.035g) and dissolve it in 1.5mL of water, and slowly add it into the above yellow solution under the condition of ice-water bath and stir thoroughly, that is, bubbles are formed, the solution turns purple, and react for 15min. The acetic acid was removed as much as possible by rotary evaporation to obtain a viscous oil, which was filtered by suction to obtain a purple solid, which was washed with dichloromethane and acetonitrile to obtain 0.4265 g of 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com