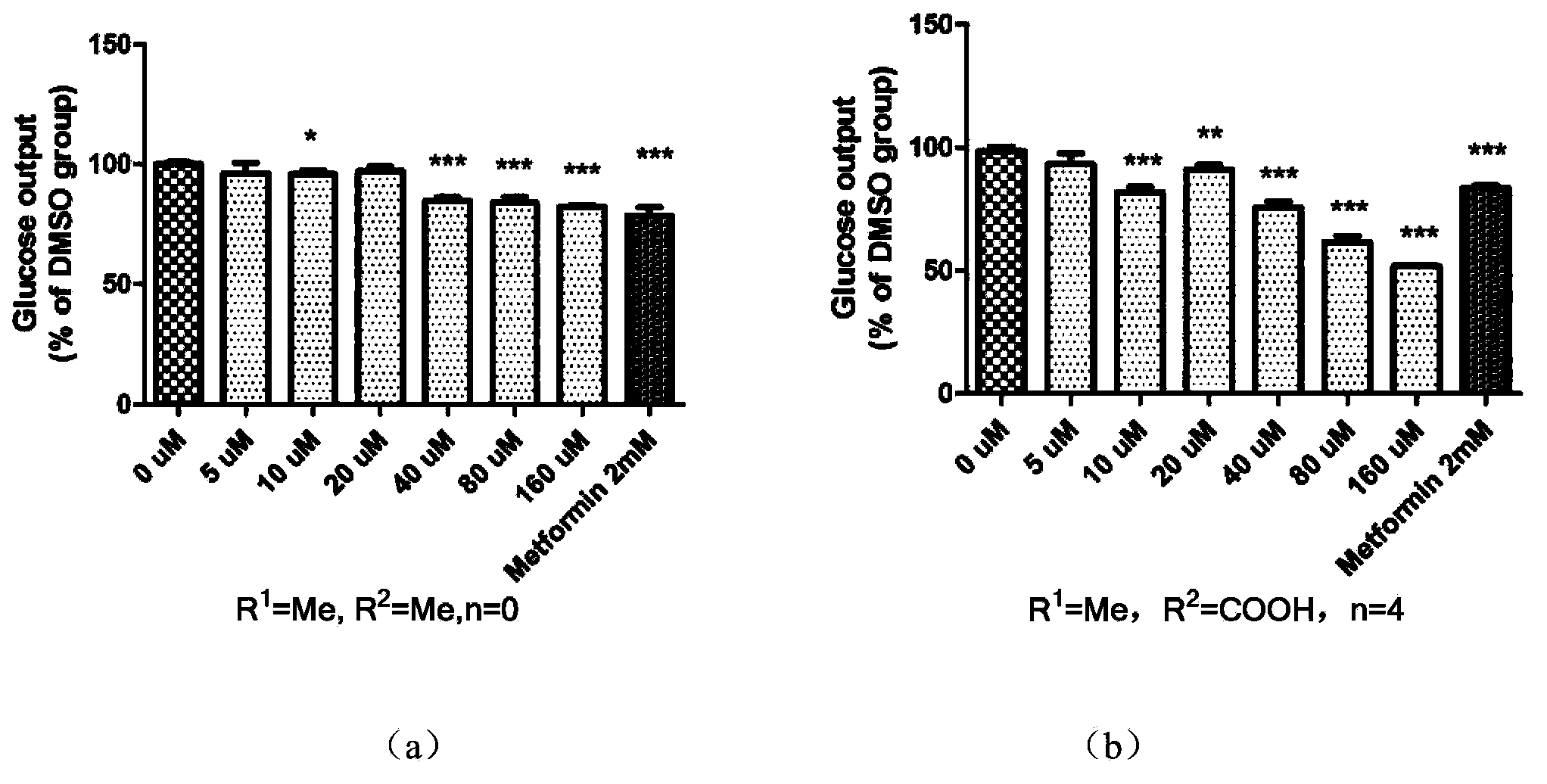
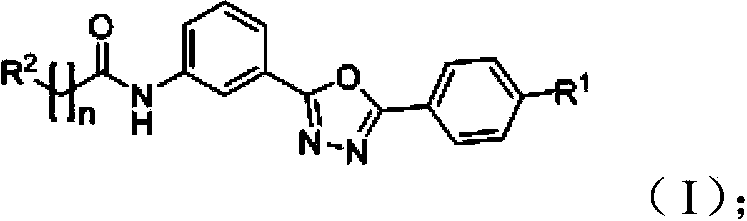
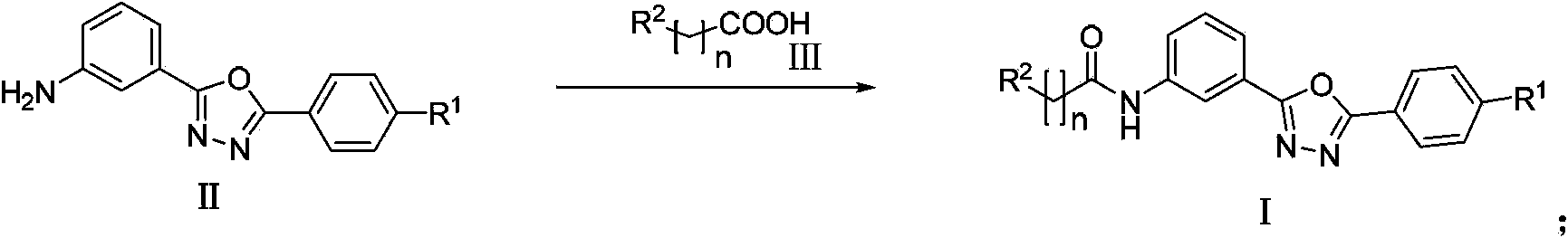Amidophenyl-1,3,4-oxadiazole compound and its preparation method and use
An amide phenyl and oxadiazole technology, applied in the field of medicine, can solve the problems of limited types of compounds, poor activity and bioavailability, etc., and achieve the effects of good druggability, reducing the risk of side effects, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Compound I of the present invention (R 1 = Me, n = 4, R 2 =OH) Preparation
[0027] The 4-substituted benzoic acid (R 1 = Me or NMe 2 ) is condensed with m-nitrobenzoic acid, dehydrated and ring-closed by phosphorus oxychloride, and then the nitro group is reduced to obtain compound II (R 1 = Me or NMe 2 ). Compound II (R) in the present invention and its various embodiments 1 = Me or NMe 2 ) is detailed in the Chinese patent application (Chinese Patent Application No. 201210405000.0).
[0028] Compound II (R 1 =Me, 0.25g, 1mmol), EDC (0.4g), HOBt (0.3g), 3-methylpyridine (0.3mL), 5-hydroxyn-pentanoic acid (1mmol) and DCM (10mL) were reacted with stirring at room temperature 24h, add DCM (20mL) and methanol (8mL) to dilute the reaction solution, followed by 5% hydrochloric acid, 5% NaOH and NaHCO 3 The solution was washed, the organic phase was collected, dried over anhydrous sodium sulfate and separated by column chromatography (DCM / MeOH=20 / 1), t...
Embodiment 2
[0029] Example 2 Compound I (R 1 = Me, n = 7, R 2 =OH) Preparation
[0030] Referring to the method shown in Example 1, wherein "5-hydroxy-n-pentanoic acid" is replaced by 7-hydroxyoctanoic acid. White solid; Yield: 33%. 1 H NMR (500MHz, DMSO-d 6 )δ1.23~1.25(m, 6H), 1.39~1.42(m, 2H), 1.60~1.62(m, 2H), 2.34(t, J=7.3Hz, 2H), 2.40(s, 3H), 3.35 ~3.37(m, 2H), 4.37(t, J=5.3Hz, 1H), 7.43(d, J=7.8Hz, 2H), 7.53(t, J=7.7Hz, 1H), 7.76(d, J=7.7Hz, 1H), 7.76(d, J=7.8Hz, 1H) 7.7Hz, 1H), 7.80(d, J=7.7Hz, 1H), 7.97(d, J=7.8Hz, 2H), 8.44(s, 1H), 10.19(s, 1H); 13 C NMR (125MHz, DMSO-d 6 )δ 171.8, 164.1, 163.8, 142.2, 140.2, 130.0 (2C), 129.9, 126.6 (2C), 123.7, 122.1, 121.1, 120.6, 116.6, 60.7, 36.5, 32.5, 28.7, 28.7, 25.4, 22.0, 21. ; HRMS (ESI): Calcd for C 23 h 28 N 3 o 3 [M+H] + , 394.2125; Found, 394.2185.
Embodiment 3
[0031] Embodiment 3 compound I (R 1 = Me, n = 3, R 2 = COOMe) preparation
[0032] Compound II (R 1 = A mixture of Me, 0.25 g, 1 mmol), EDC (0.4 g), HOBt (0.3 g), 3-picoline (0.3 mL), monomethyl succinate (1 mmol) and DCM (10 mL) was stirred at room temperature Reacted for 24h, added DCM (20mL) and methanol (8mL) to dilute the reaction solution, followed by 5% hydrochloric acid, 5% NaOH and NaHCO 3 The solution was washed, and the organic phase was collected, dried over anhydrous sodium sulfate and separated by column chromatography ((DCM / EA=10 / 1) to obtain the product I (R 1 = Me, n = 3, R 2 =COOMe) 0.18g. White solid; Yield: 50%. 1 H NMR (400MHz, CDCl 3 )δ2.04~2.11(m, 2H), 2.39(s, 3H), 2.45(t, J=7.3Hz, 2H), 2.54(t, J=7.3Hz, 2H), 3.65(s, 3H), 7.26(d, J=8.0Hz, 2H), 7.41(t, J=8.0Hz, 1H), 7.79(d, J=7.8Hz, 1H), 7.88(d, J=7.8Hz, 1H), 7.93( d, J=8.0Hz, 2H), 8.33(s, 1H), 8.81(s, 1H); 13 C NMR (100MHz, CDCl 3 )δ 173.6, 171.2, 164.7, 164.1, 142.3, 139.1, 129.7(2C), 129.6, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com