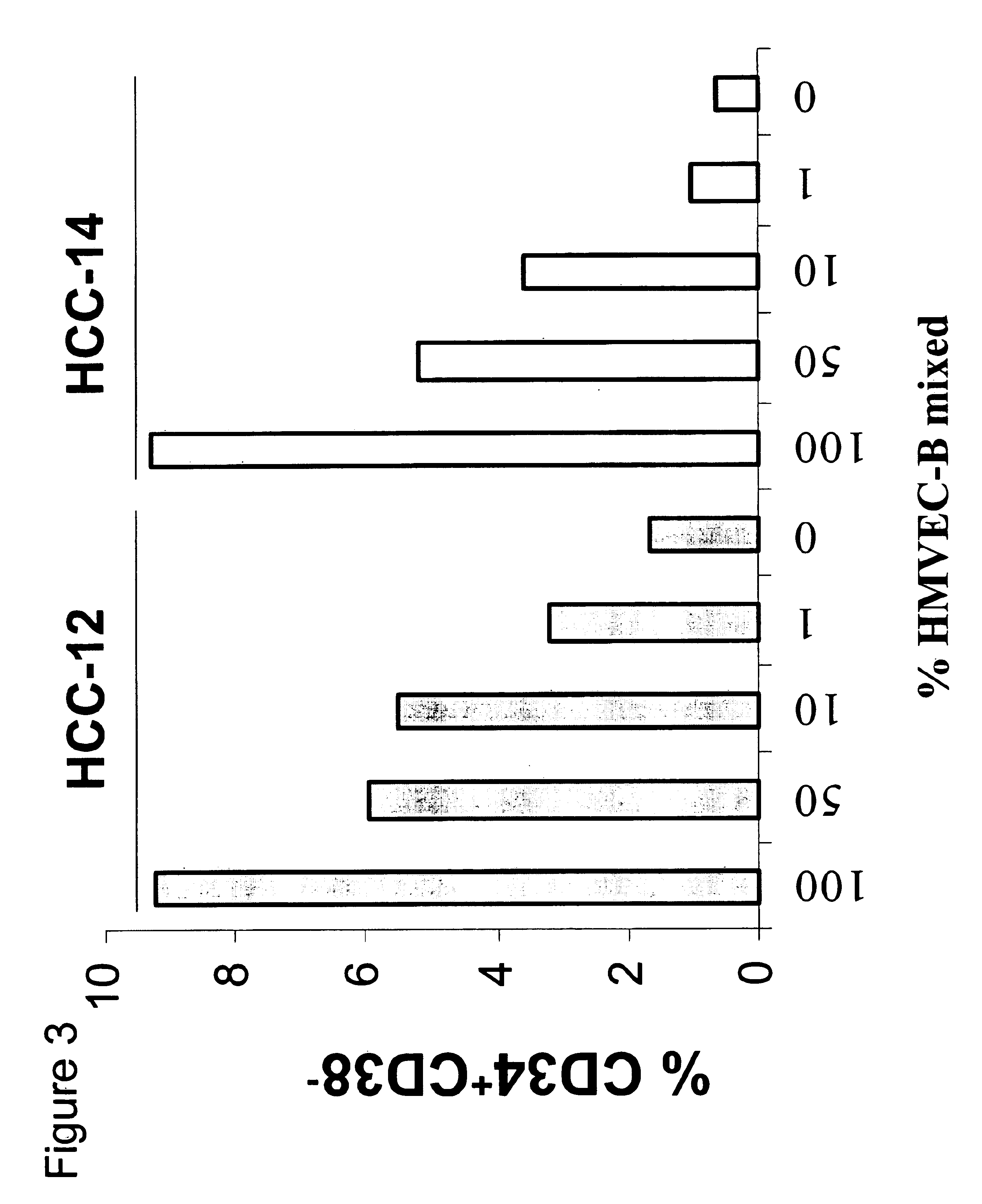
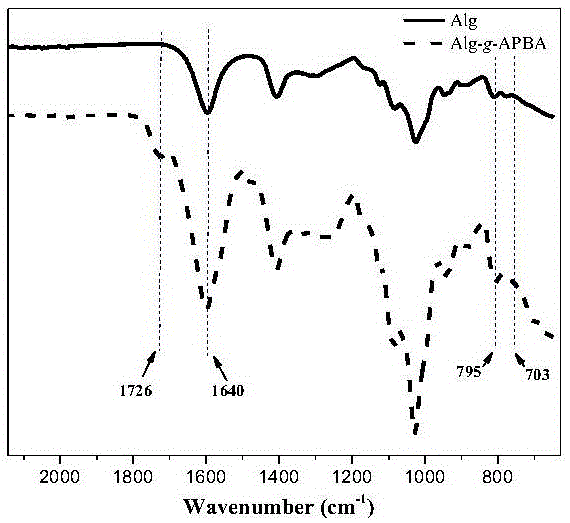
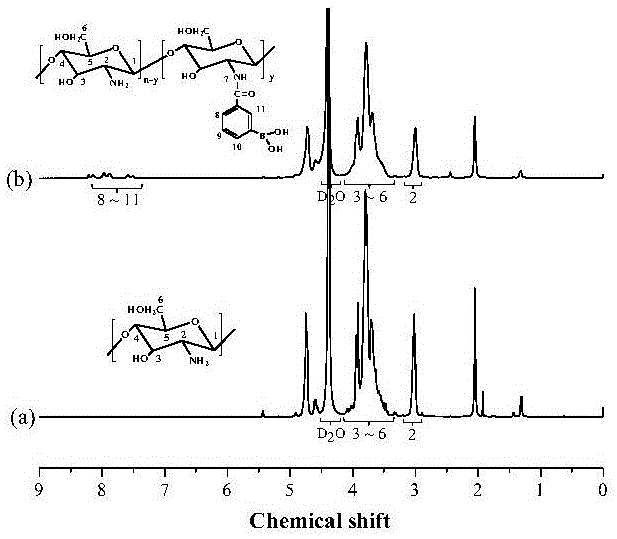
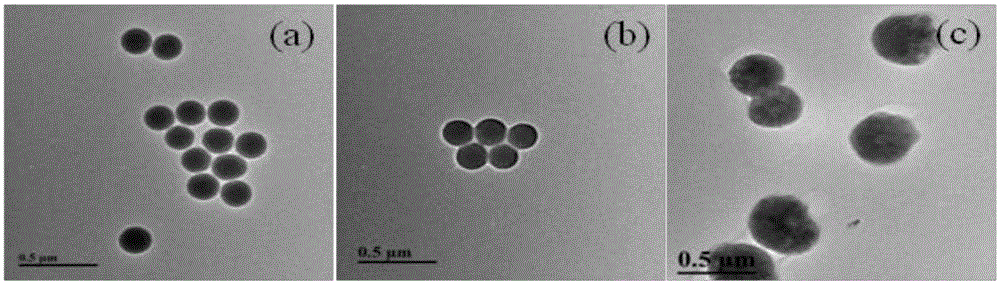
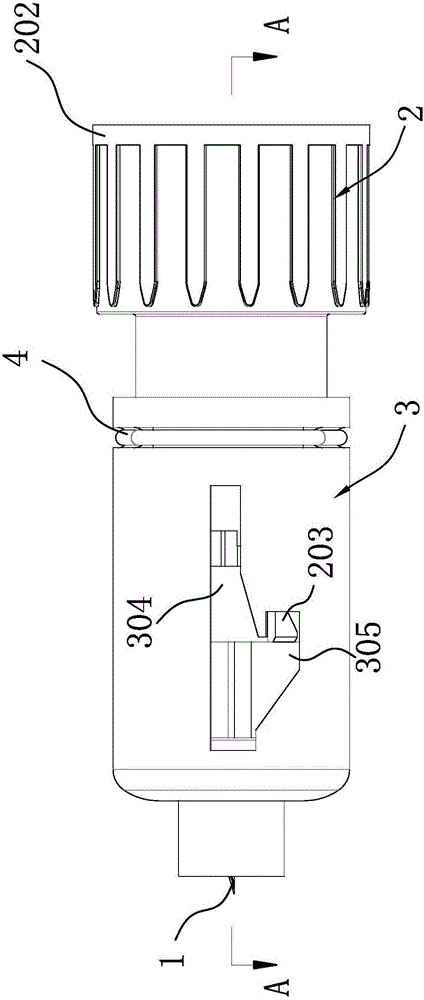
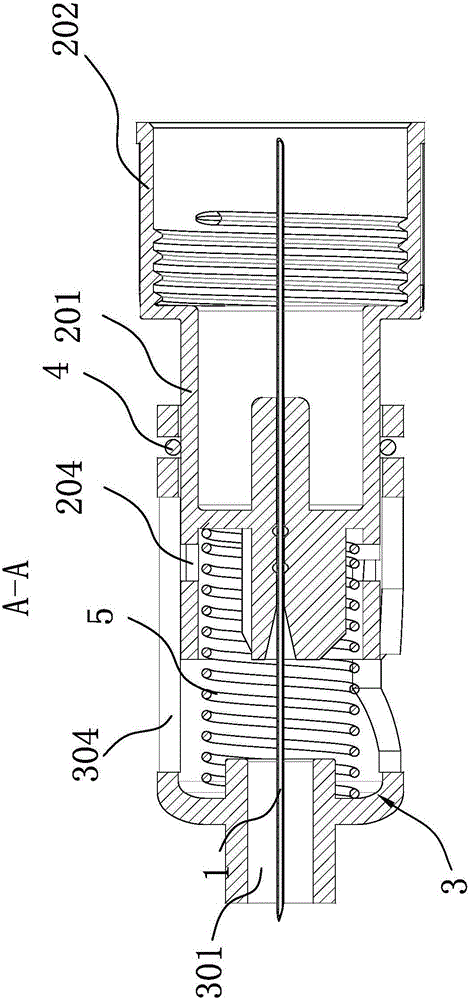
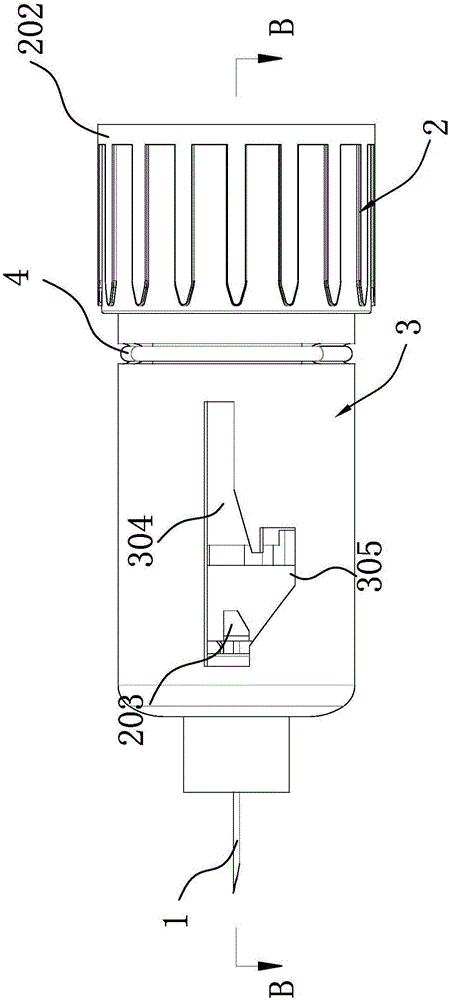
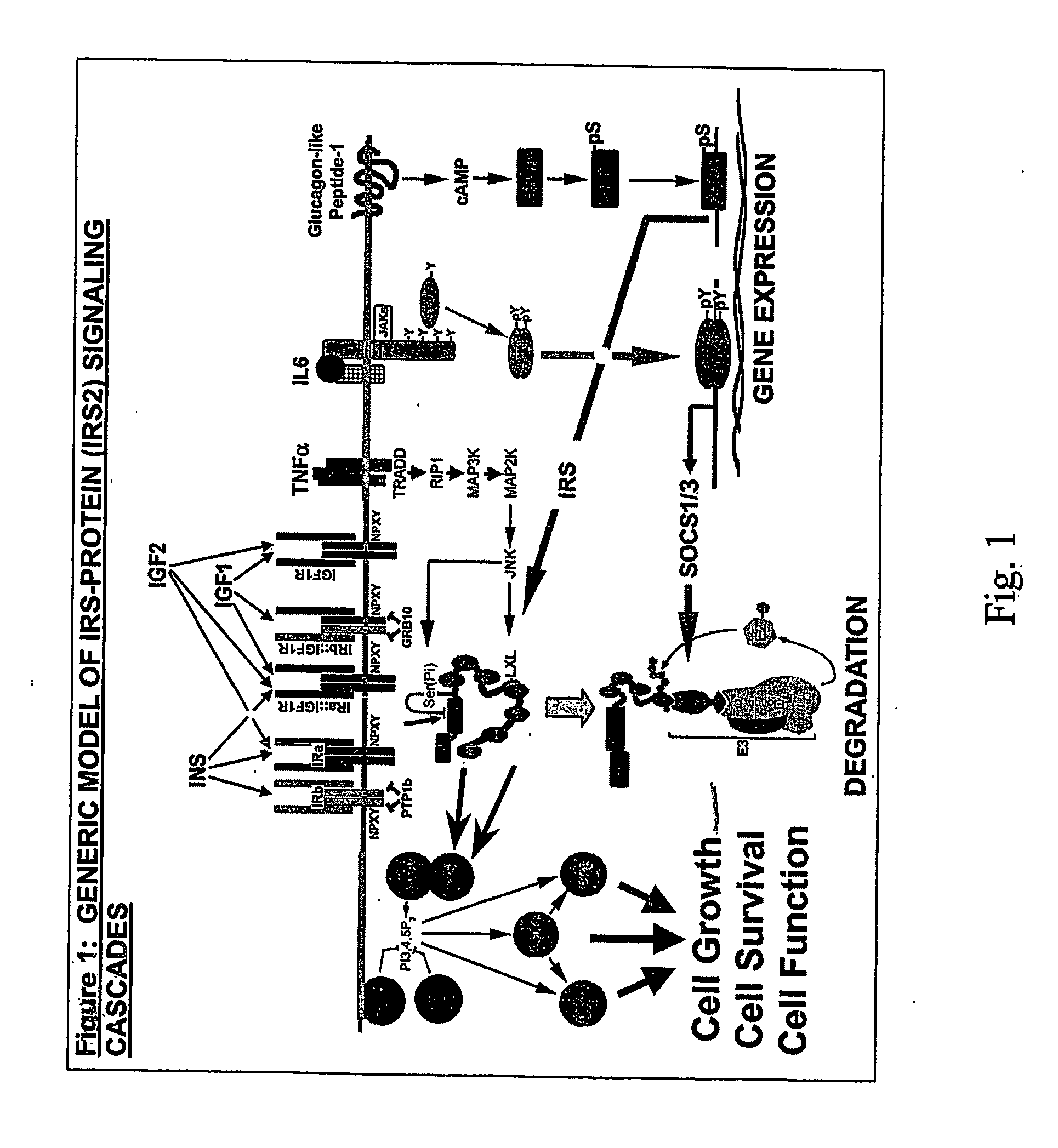
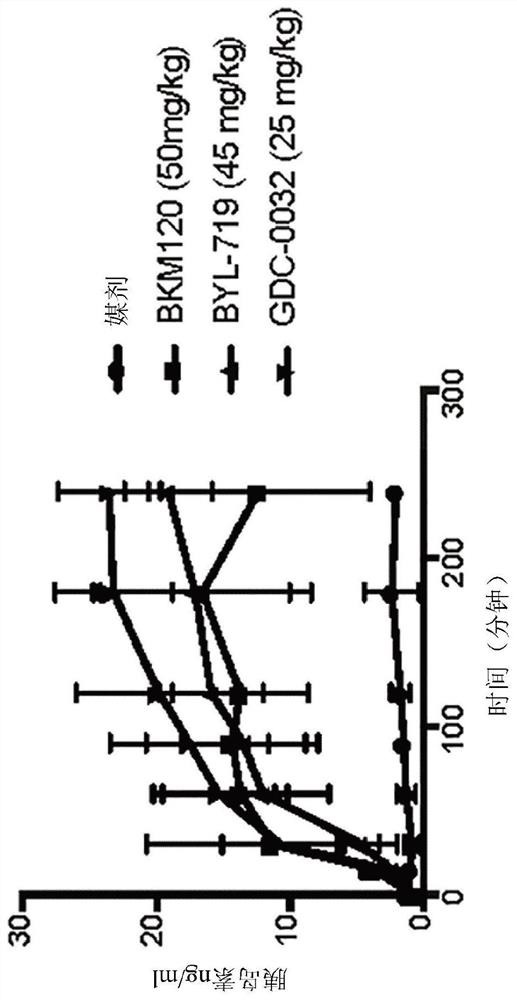
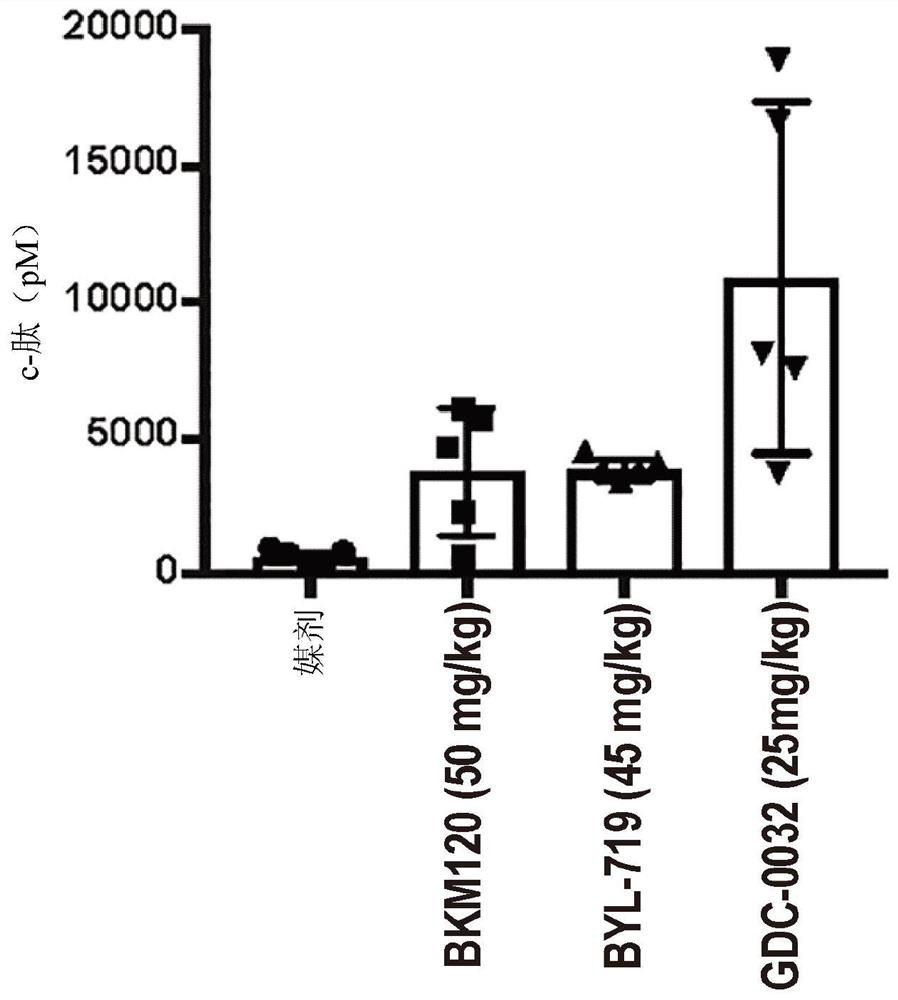
Patents
Literature
85 results about "Use insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Autism treatment
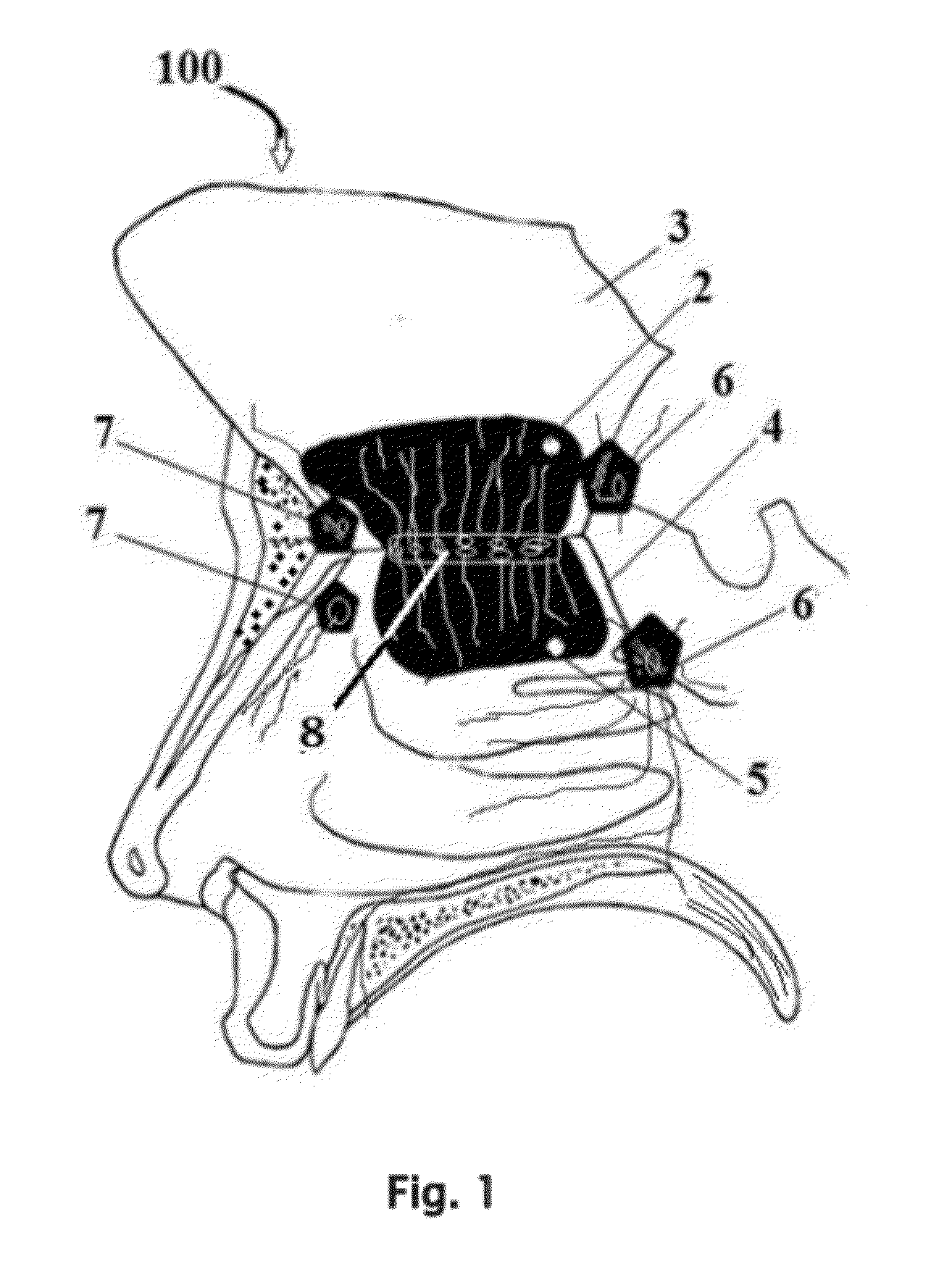
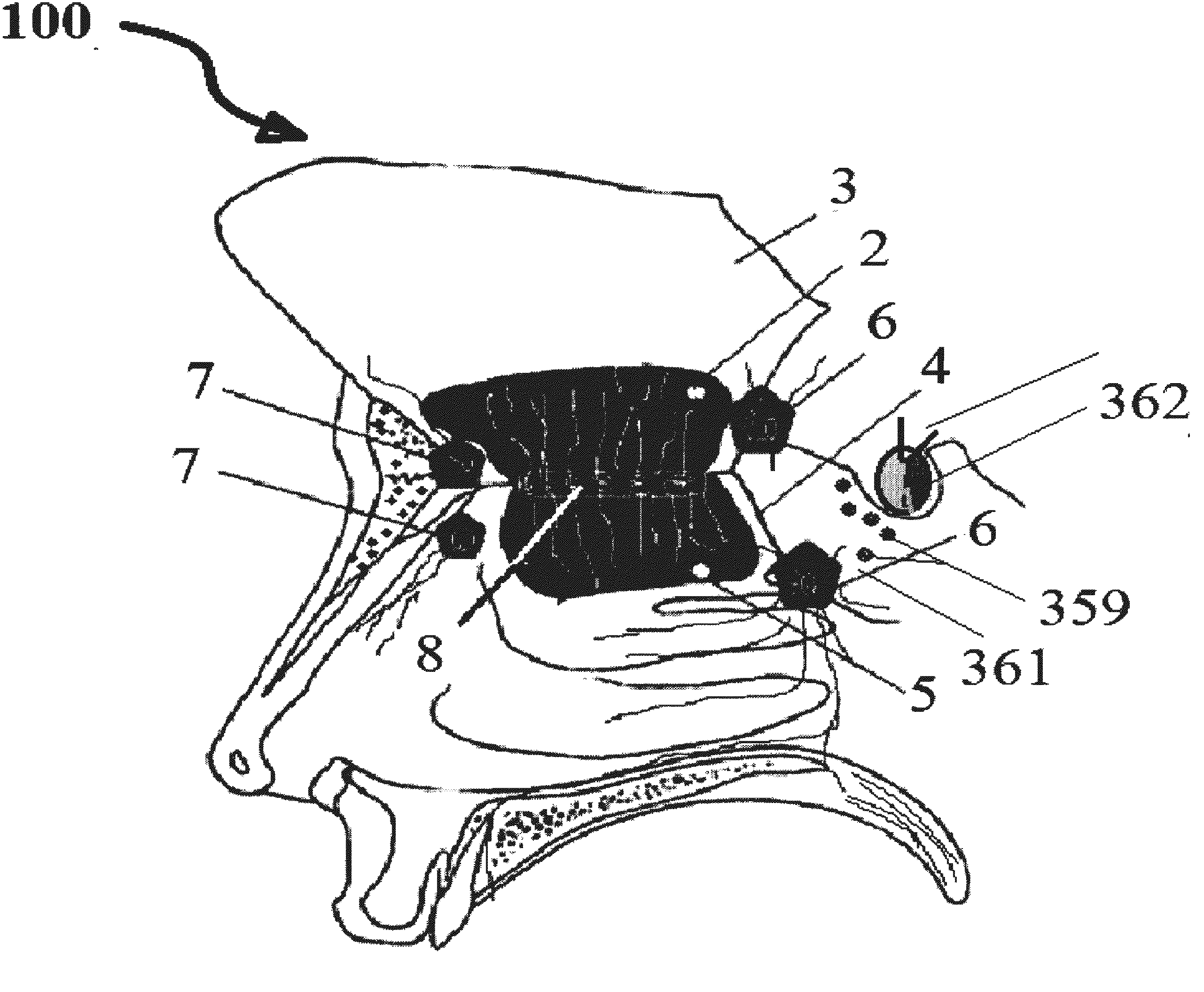
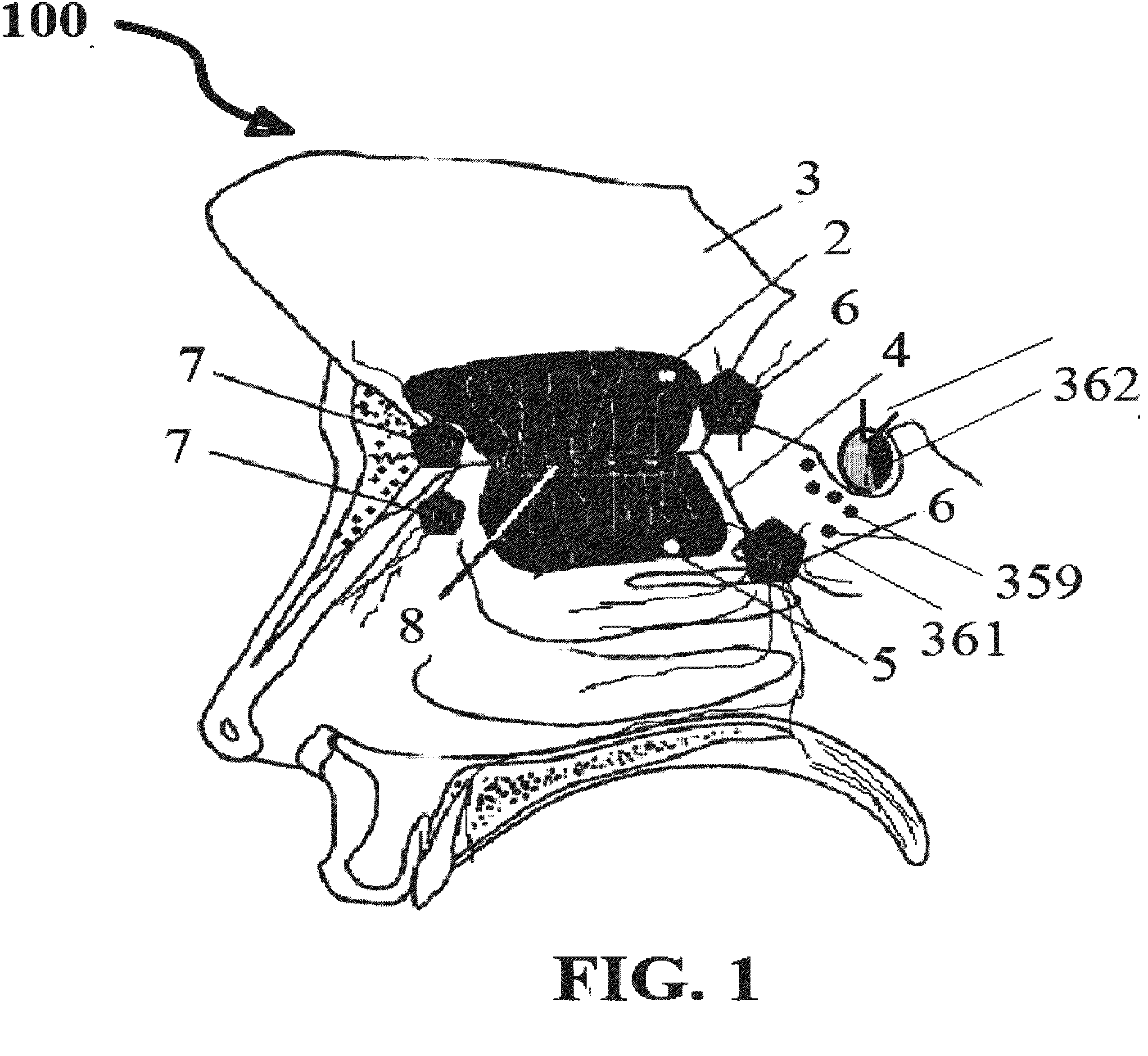
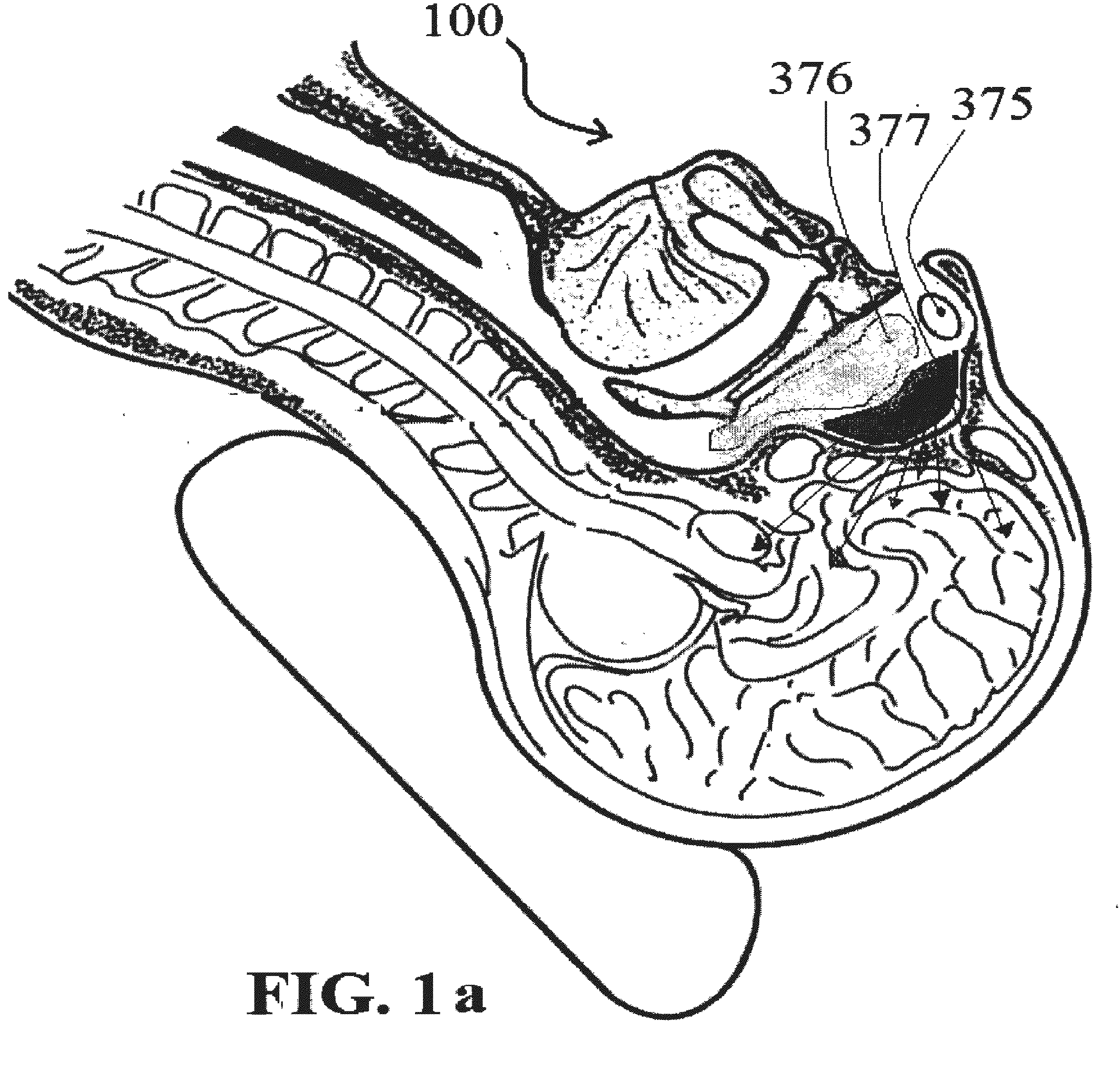
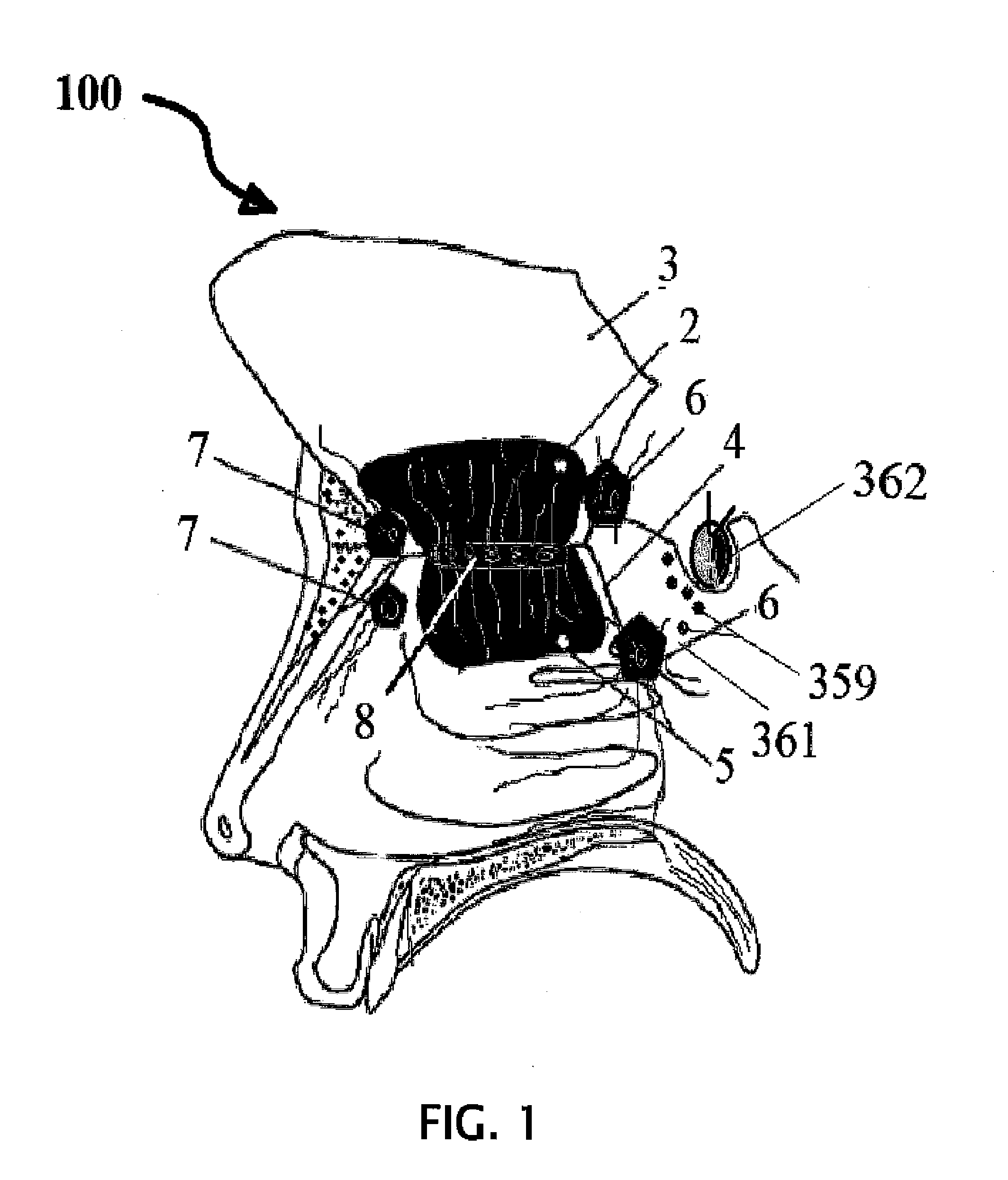
InactiveUS20120128683A1Treat and prevent associated lossMinimally invasiveNervous disorderPeptide/protein ingredientsMedicineNose
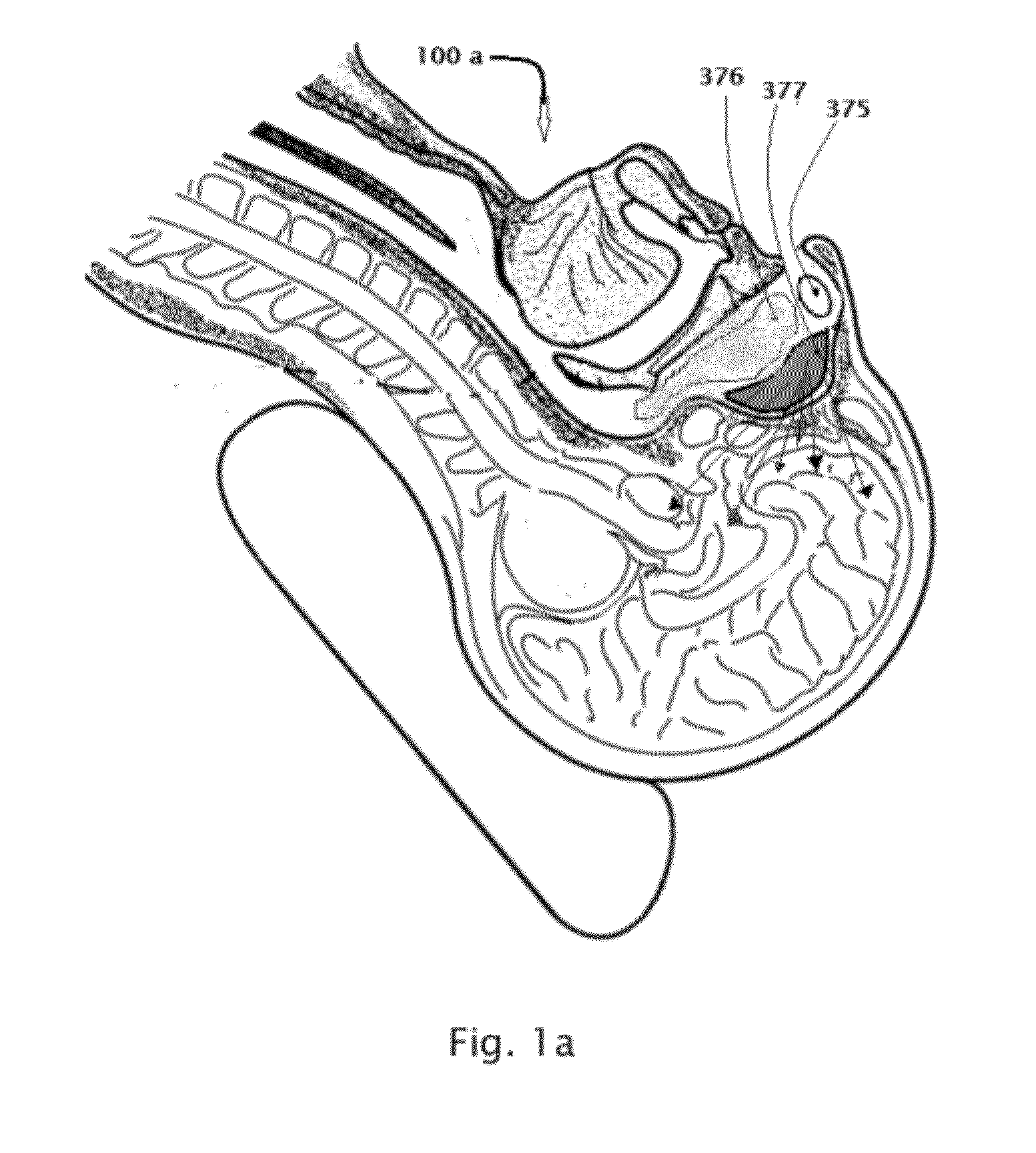
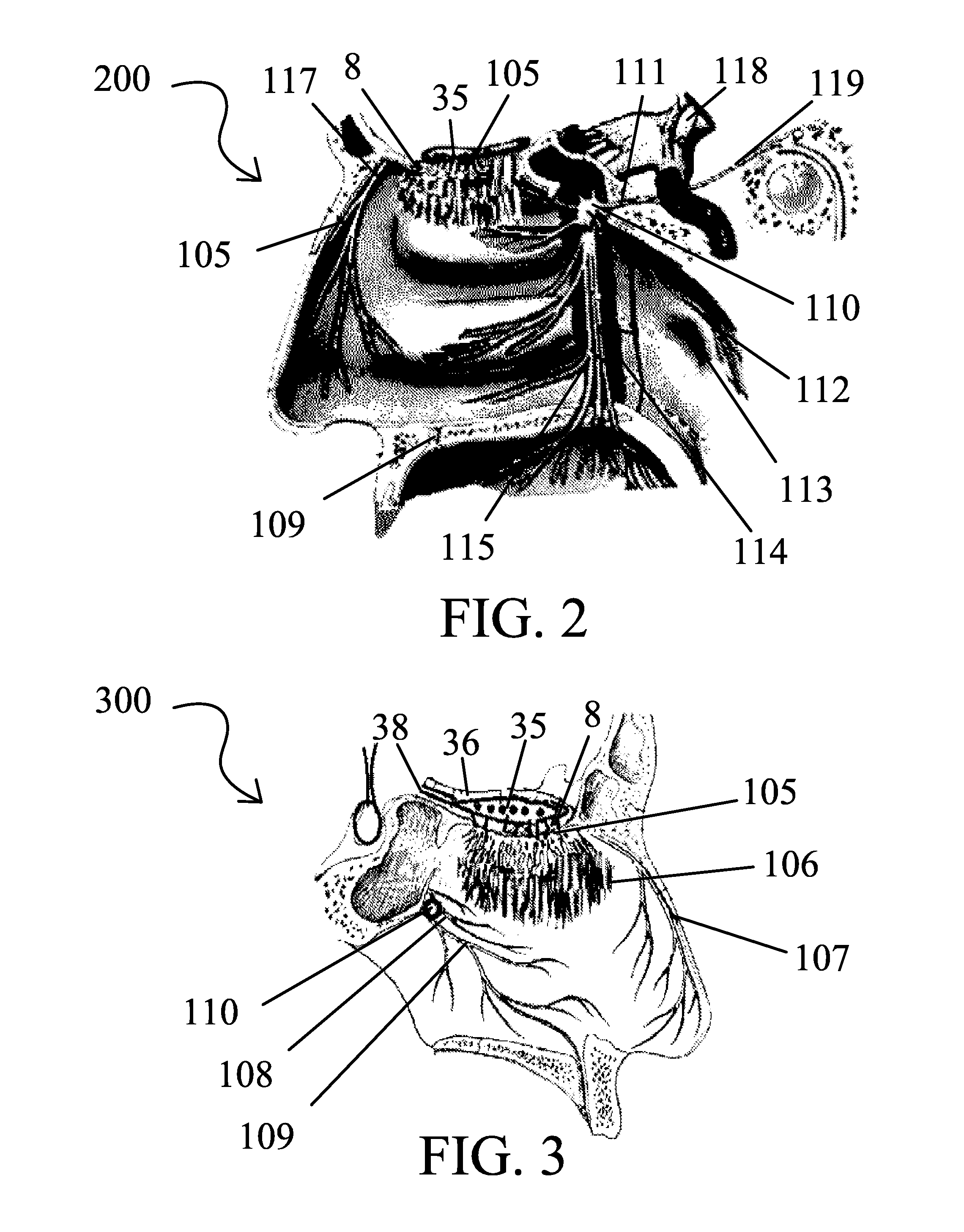
A safe and effective treatment to curtail and cure autism spectrum disorders has been described in this invention using insulin, IGF-1, with multiple known adjuvant therapeutic agents, as well as other pharmaceutical, biochemical, nurticeuticals, and biological agents or compounds delivered through the olfactory mucosal region of the nose and external auditory meatus.
Owner:SHANTHA TOTADA R
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
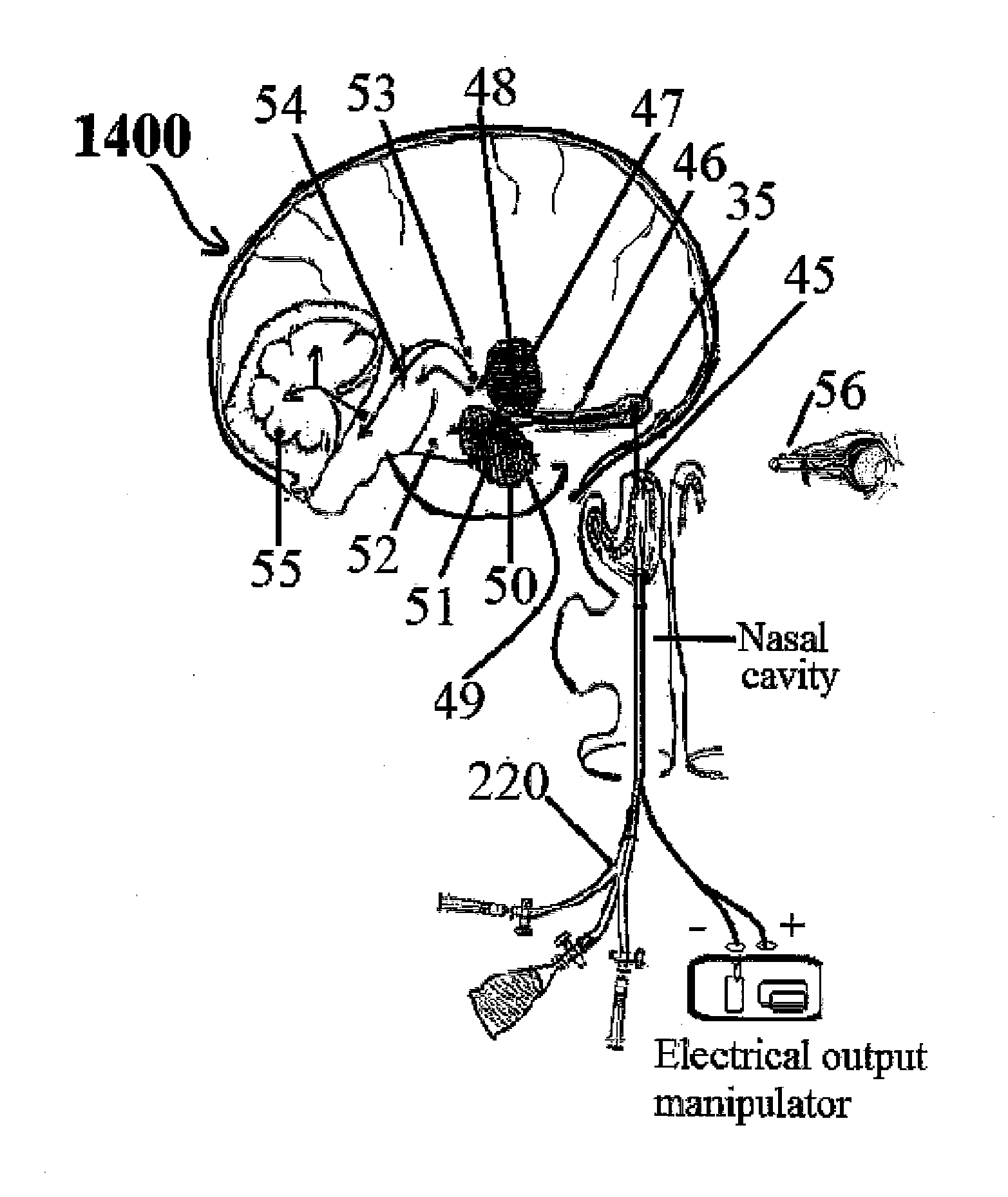
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20140012182A1Large deliveryAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Medical infusion pump capable of learning bolus time patterns and providing bolus alerts
ActiveUS20050278073A1Drug and medicationsPharmaceutical delivery mechanismInsulin pumpEmergency medicine
An apparatus and method are disclosed for improving a medical infusion pump. Users of medical infusion pumps, such as insulin pumps, require a bolus of a medication at predicable times of the day, such as at or near mealtimes for insulin pumps. The disclosed medical infusion pump determines bolus time intervals during which boluses are usually taken, and, alerts the user at one or more calculated alert times during an active bolus time interval when a bolus has not yet been delivered during the active bolus time interval. Advantageously, a different set of bolus time intervals are determined by day of week, to accommodate, for example, different bolus patterns during weekends versus weekdays.
Owner:TANDEM DIABETES CARE INC
Rabies cure
InactiveUS20110020279A1Inhibit rabies virus multiplicationAvoid spreadingSsRNA viruses negative-senseOrganic active ingredientsSubarachnoid spacePresent method
This invention is for a method of treatment of rabies once the patient develops signs and symptoms of rabies with the intent to save the patients from death and disability using insulin combined with various anti rabies viral therapeutic, pharmaceutical, biochemical, and biological agents or compounds with added supportive therapies administered through OM, SAS, IVB, IV, and IA routes. An embodiment provides devices for intranasal delivery of therapeutic agents to olfactory mucosal area. Another embodiment uses the technology to deliver the therapeutic, pharmaceutical, biochemical, and biological agents or compounds to the subarachnoid space and ventricular system by using continuous catheters and Ommaya reservoir at the same time. The present method incorporates breaking the blood brain barrier to allow the entry of the anti rabies therapeutic agents into the neuropile. Additionally, an embodiment incorporates cooling of the brain and inducing hibernation to preserve the brain from damage due to rabies.
Owner:SHANTHA TOTADA R
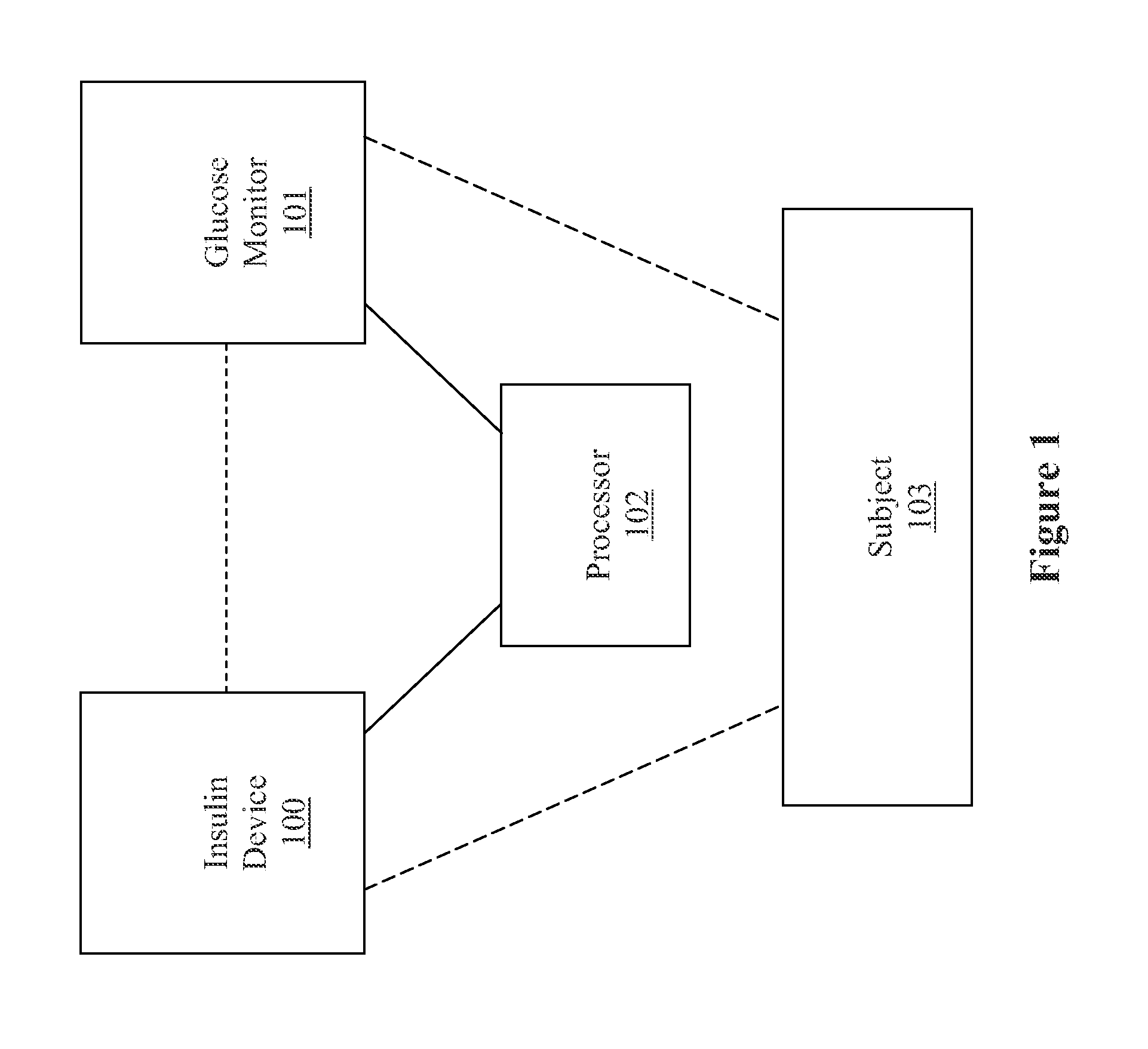
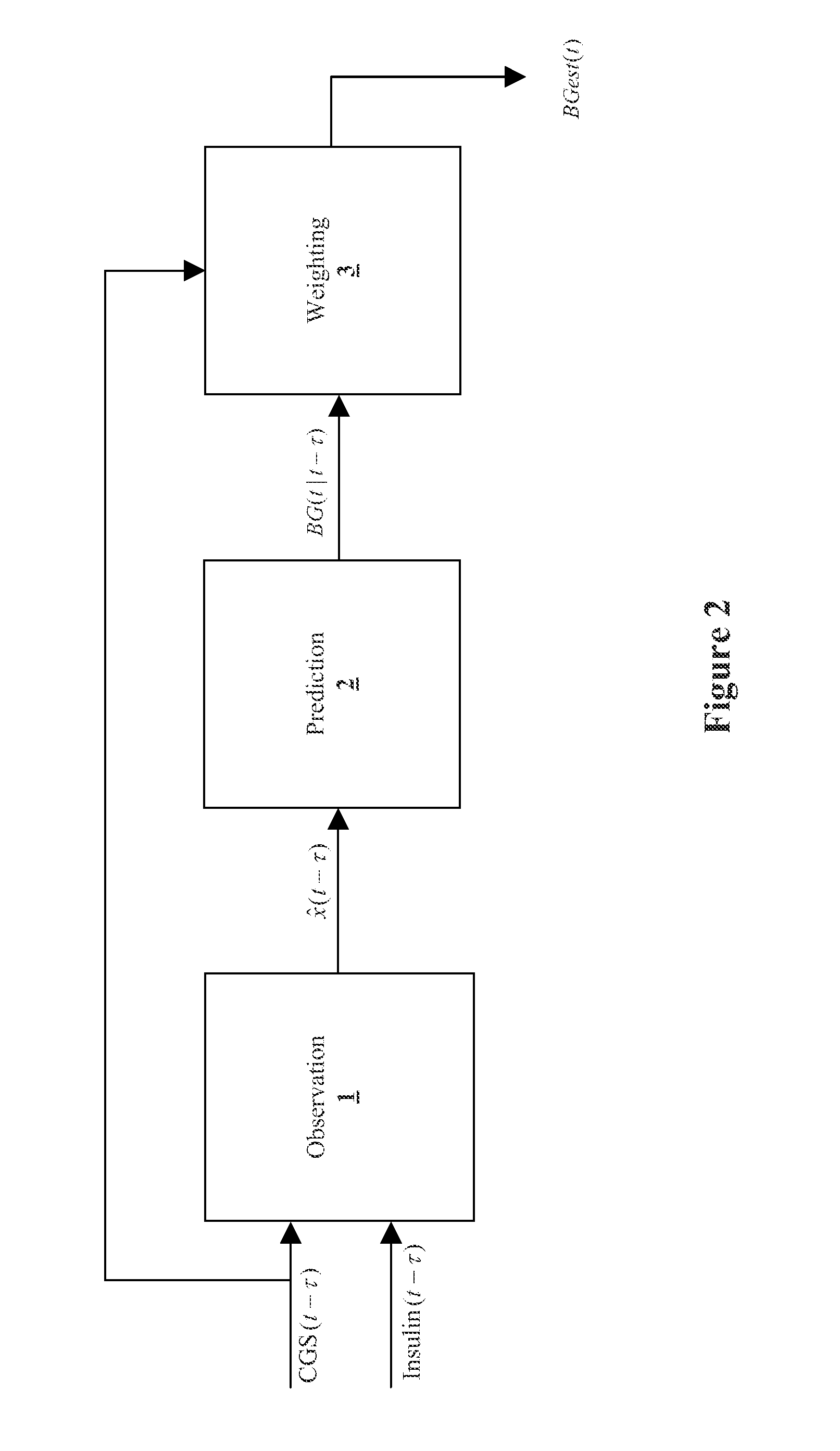
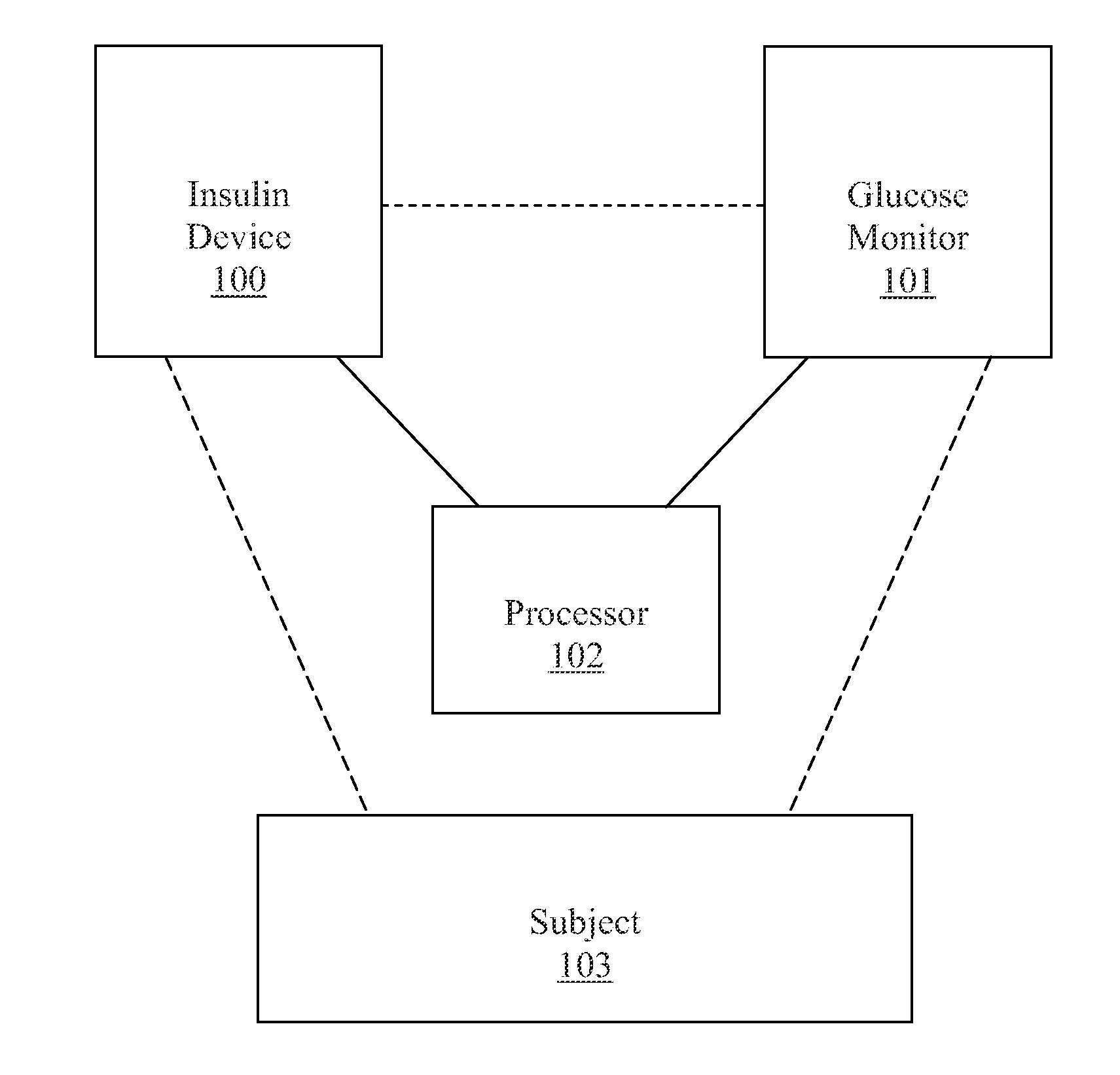
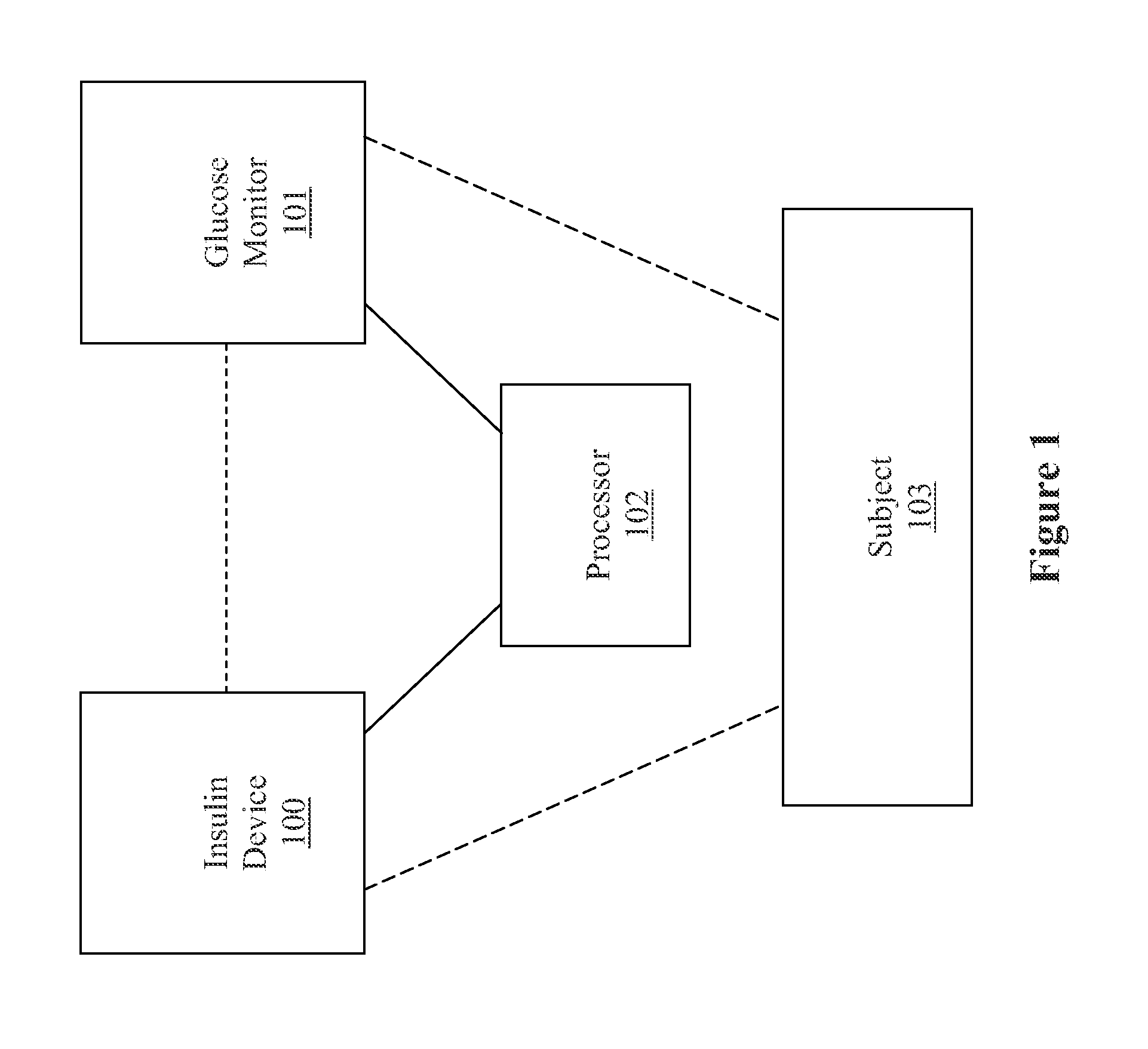
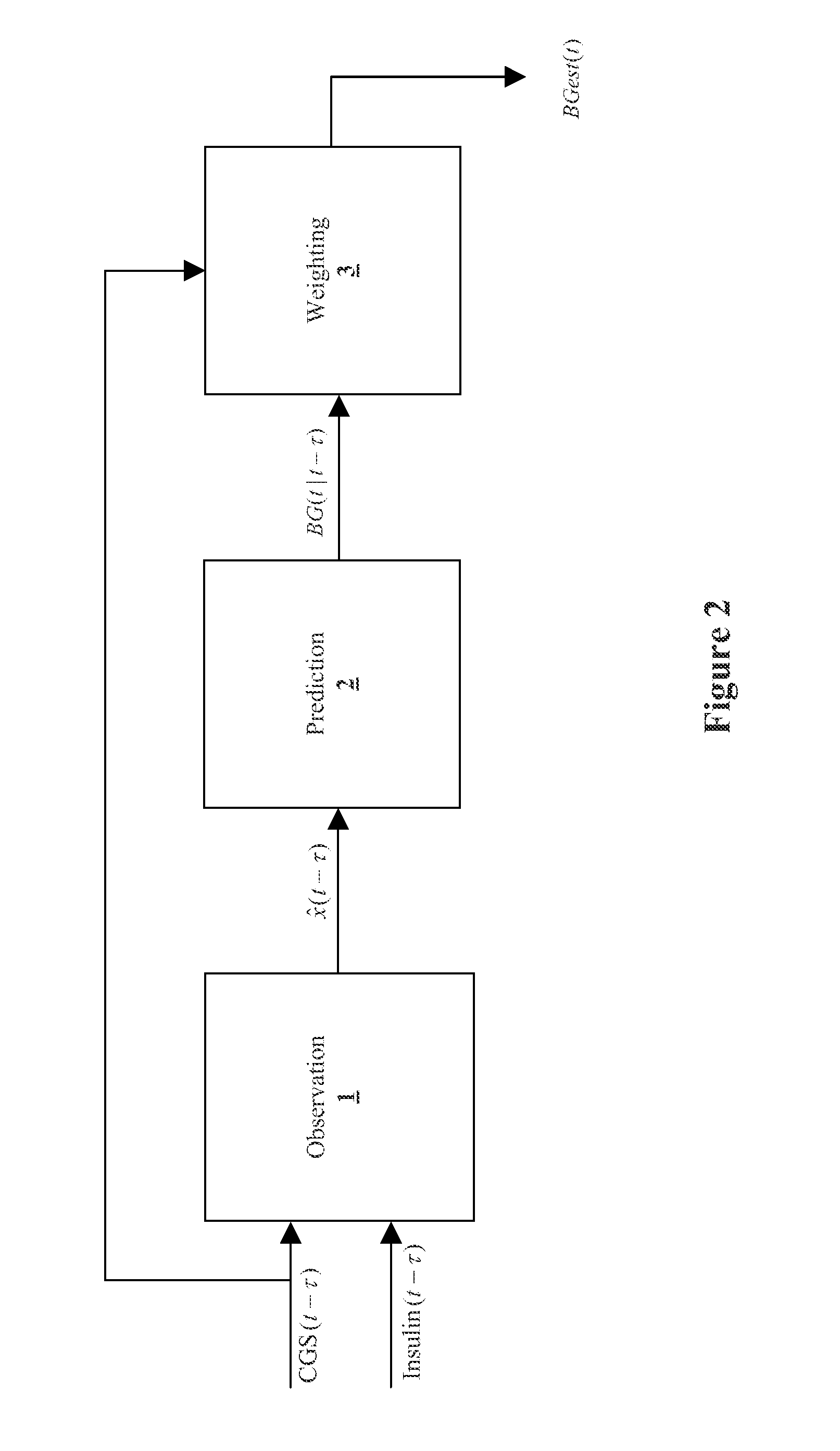
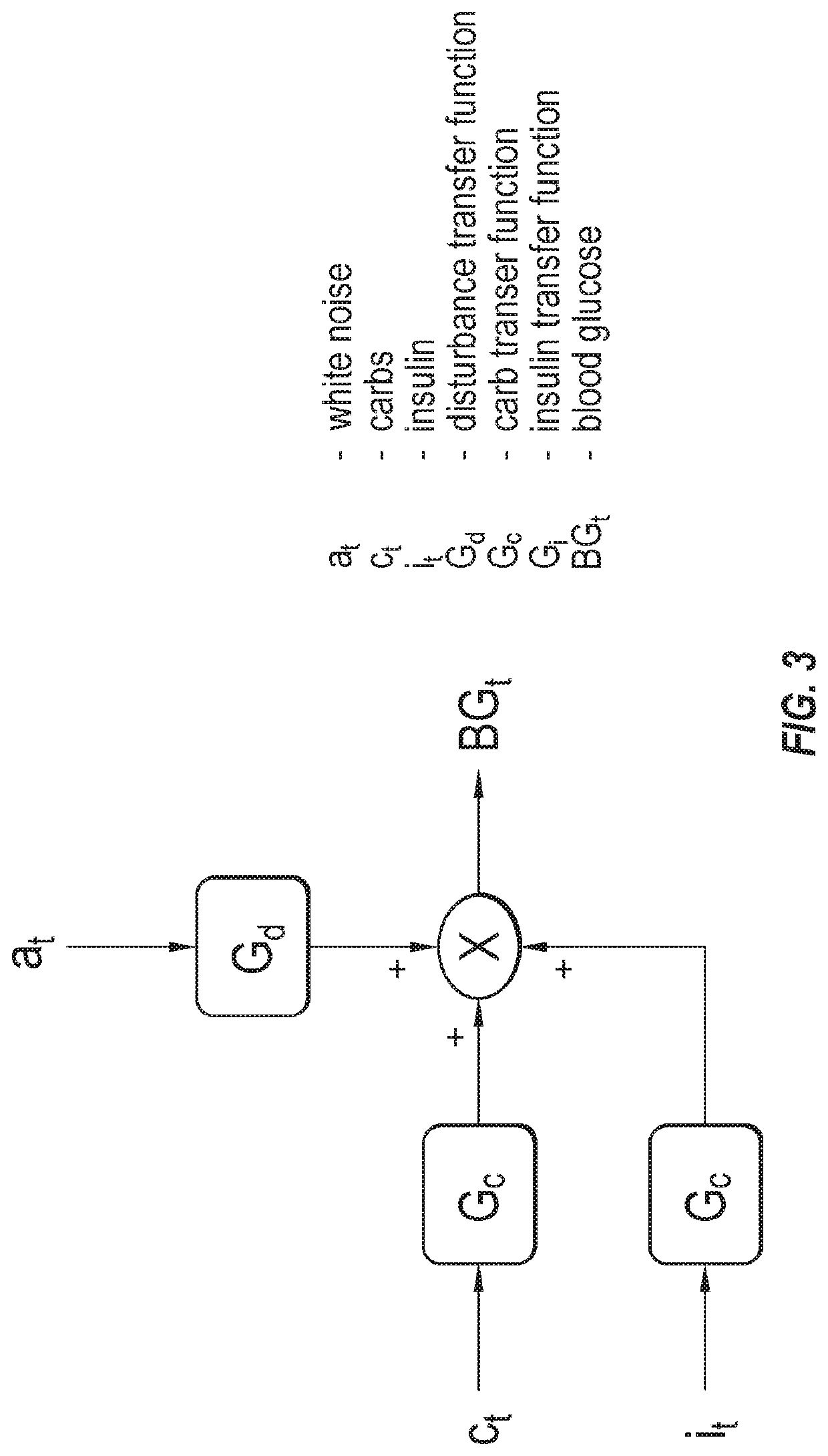
Method, system, and computer program product for improving the accuracy of glucose sensors using insulin delivery observation in diabetes
ActiveUS9398869B2Improve accuracyImprove sensor accuracyOther blood circulation devicesPressure infusionGlucose sensorsInsulin pump
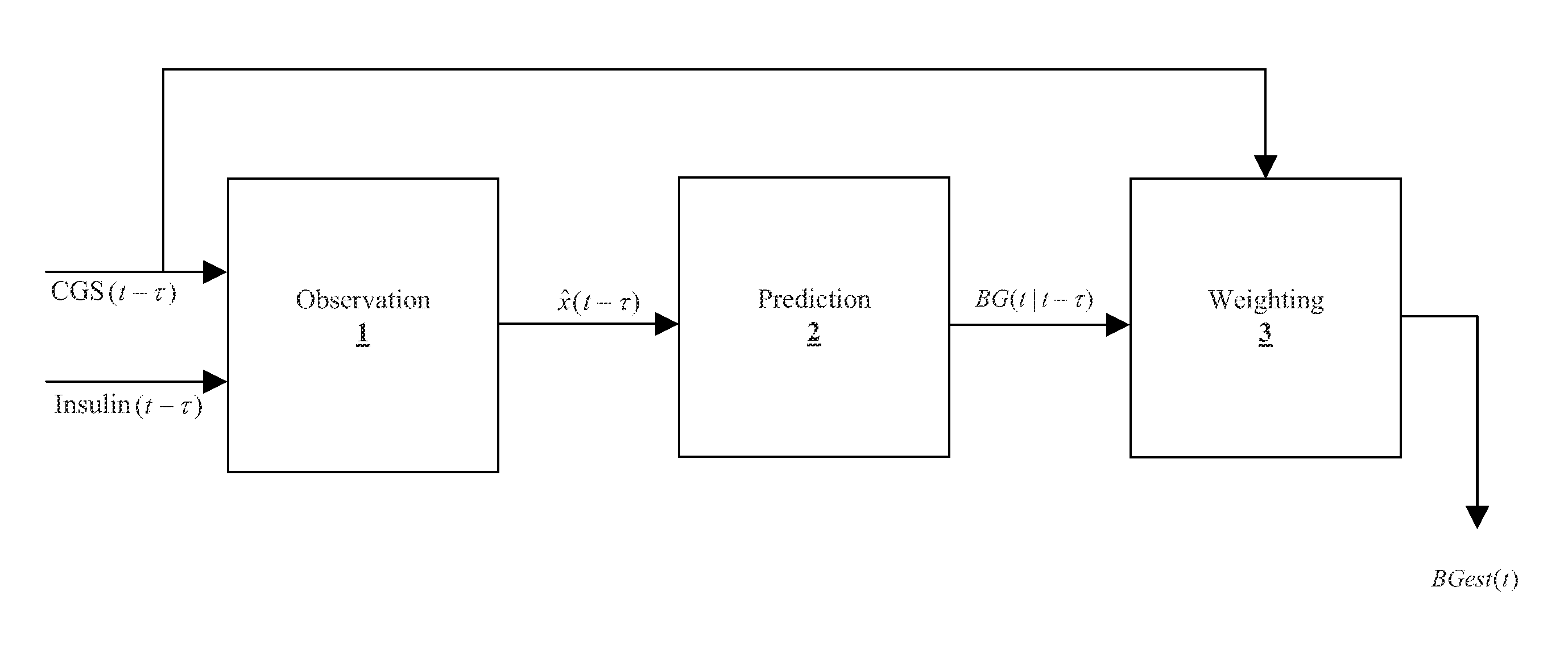
Method and System for providing a signal from an insulin pump, artificial pancreas, or another insulin delivery device as a source of information for improving the accuracy of a continuous glucose sensor (CGS). The effect of using insulin information to enhance sensor accuracy is most prominent at low blood glucose levels, i.e. in the hypoglycemic range, which is critical for any treatment. A system for providing a filtering / state estimation methodology that may be used to determine a glucose state estimate at time t-τ. The estimation may be extrapolated to some future time t and then the extrapolated value is used to extract the blood glucose component. The blood glucose component of the extrapolation and the output of the CGS are weighted and used to estimate the blood glucose level of a subject.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Compositions and methods for treating articular cartilage disorders
InactiveUS7141545B2Increase volumeEasy maintenancePeptide/protein ingredientsSkeletal disorderInsulin-like growth factorDisease
A method for treating mammalian articular cartilage disorders, more particularly osteoarthritis, and trauma-related cartilage injuries using insulin-like growth factor I (IGF-I) is provided. The method comprises increasing the amount of IGF-I at the diseased or injured articular site to a therapeutically effective level that is capable of maintenance and / or regeneration of cartilage, which is beneficial to the long-term treatment of osteoarthritis and trauma-related injuries to cartilage tissues. In one embodiment of the invention, single doses of at least 0.01 mg of pharmaceutically effective IGF-I are administered intermittently such that the duration of time off of therapy is greater than the time on therapy, more preferably with a frequency of administration of about once per week or less.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Method, system, and computer program product for improving the accuracy of glucose sensors using insulin delivery observation in diabetes
ActiveUS20130079613A1Improve accuracyImprove sensor accuracyOther blood circulation devicesPressure infusionGlucose sensorsInsulin pump
Method and System for providing a signal from an insulin pump, artificial pancreas, or another insulin delivery device as a source of information for improving the accuracy of a continuous glucose sensor (CGS). The effect of using insulin information to enhance sensor accuracy is most prominent at low blood glucose levels, i.e. in the hypoglycemic range, which is critical for any treatment. A system for providing a filtering / state estimation methodology that may be used to determine a glucose state estimate at time t-τ. The estimation may be extrapolated to some future time t and then the extrapolated value is used to extract the blood glucose component. The blood glucose component of the extrapolation and the output of the CGS are weighted and used to estimate the blood glucose level of a subject.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
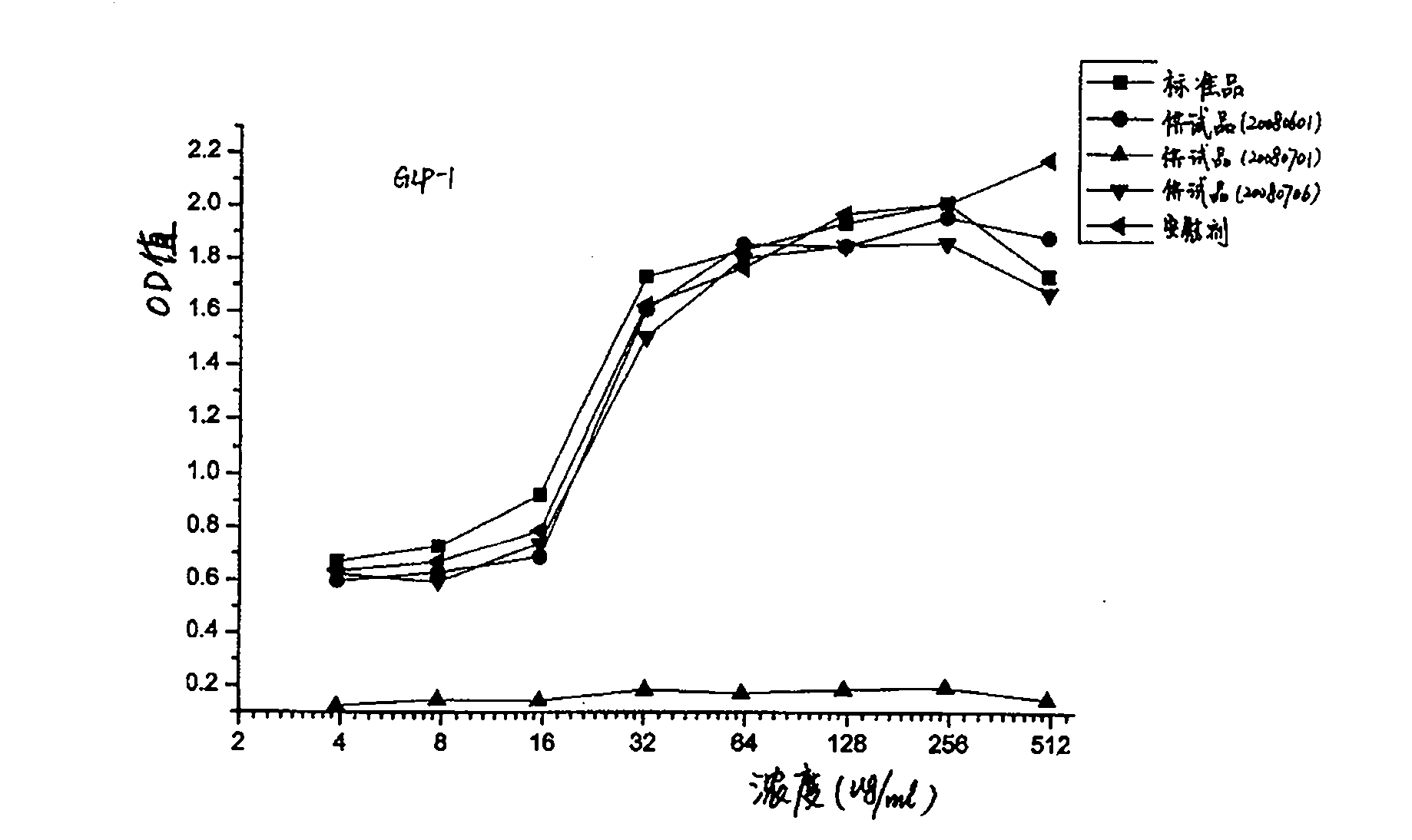
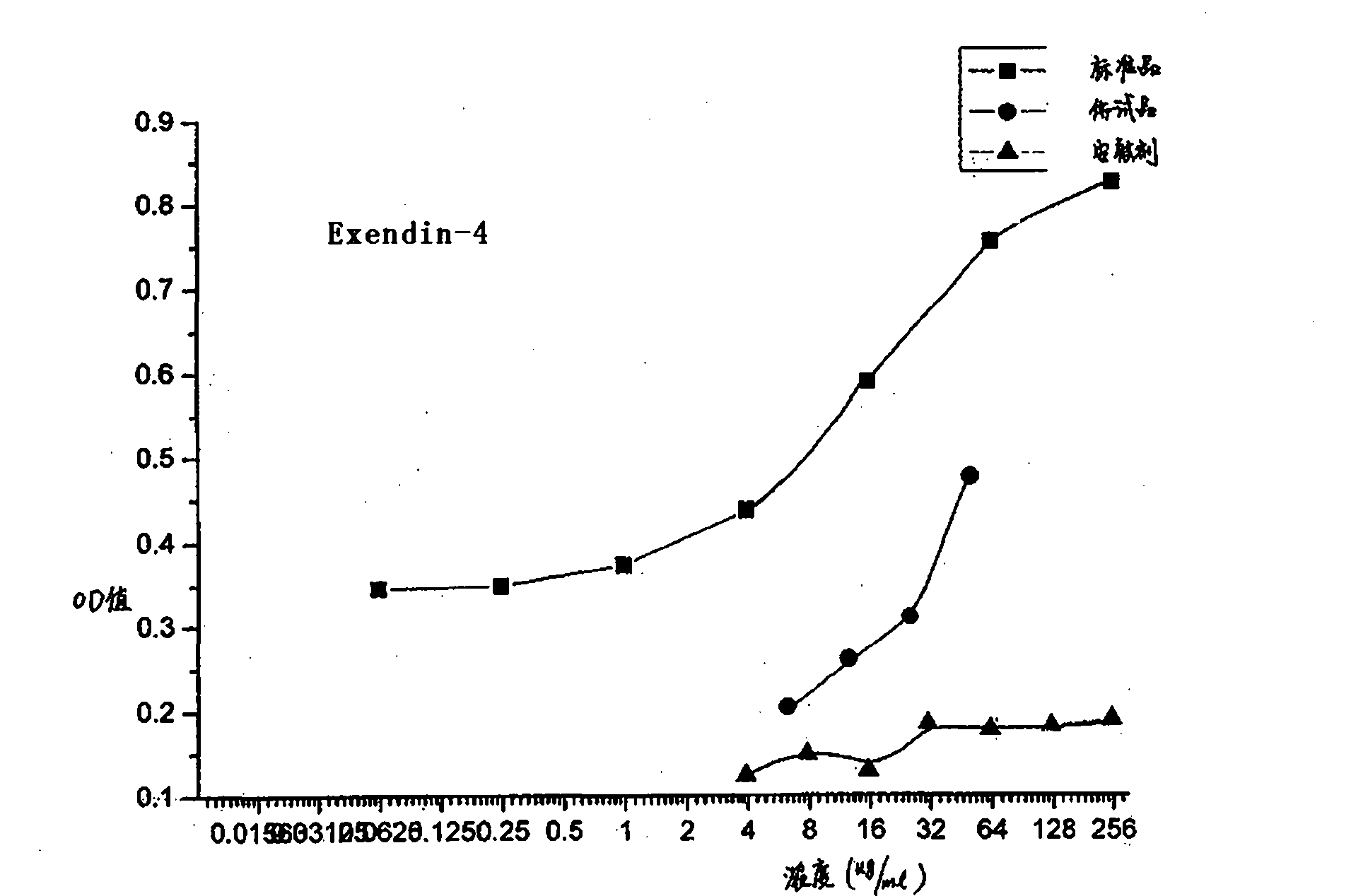
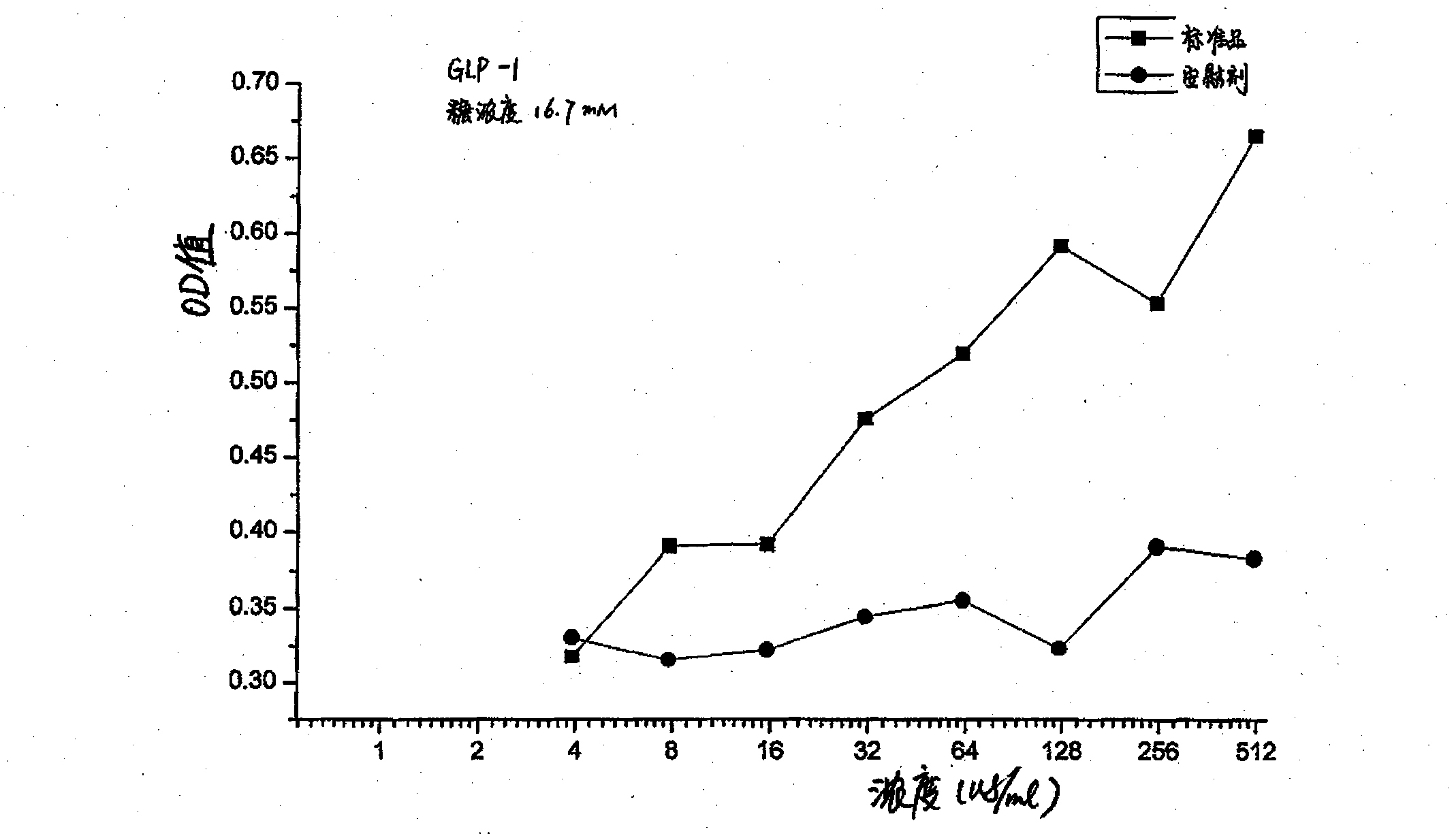
Method for testing bioactivities of GLP-1 receptor agonist
ActiveCN101798588AMicrobiological testing/measurementColor/spectral properties measurementsAgonistLength wave
The invention relates to a method for testing bioactivities, in particular to a method for testing the bioactivities of a GLP-1 receptor agonist. Based on the function of the GLP-1 receptor agonist which can promote mouse insulinoma cells (Min-6) to secrete the insulin, the method comprises the steps of: using insulin quantitatively secreted by a mouse / rat insulin kit to improve the precision of determination through a series of condition optimization processes, and testing the absorbency at the wavelength of 450 nm and the reference wavelength of 590 nm to obtain the effect curve that the GLP-1 receptor agonist induces the Min-6 cells to secrete the insulin; and finally testing the bioactivities of the GLP-1 receptor agonist on the basis of the effect curve.
Owner:SHANGHAI HUAYI BIO LAB CO LTD +1
Modulation of primary stem cell differentiation using an insulin-like growth factor binding protein
InactiveUS6841386B2Limited to differentiationReduce concentrationPeptide/protein ingredientsMicrobiological testing/measurementUse insulinProtein C
The present invention features methods of modulating primary stem cell differentiation in culture by altering the endogenous activity of an insulin-like growth factor.
Owner:VIACELL
Method for preparing multi-layer bio-based vesica capable of releasing insulin
ActiveCN105078890AAchieve controlled releaseImprove stabilityPeptide/protein ingredientsMetabolism disorderMicrosphereBiocompatibility Testing
Owner:JIANGNAN UNIV
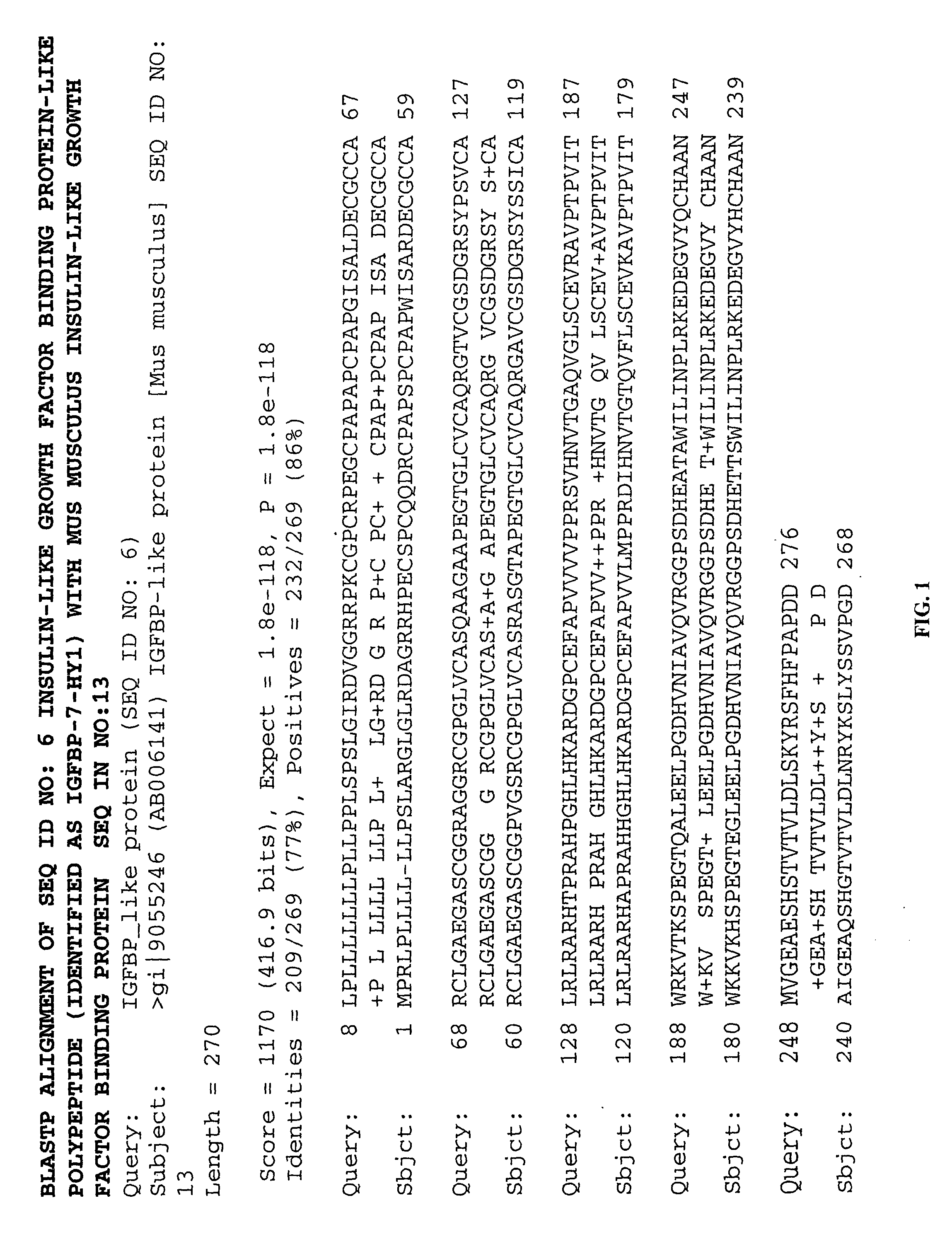
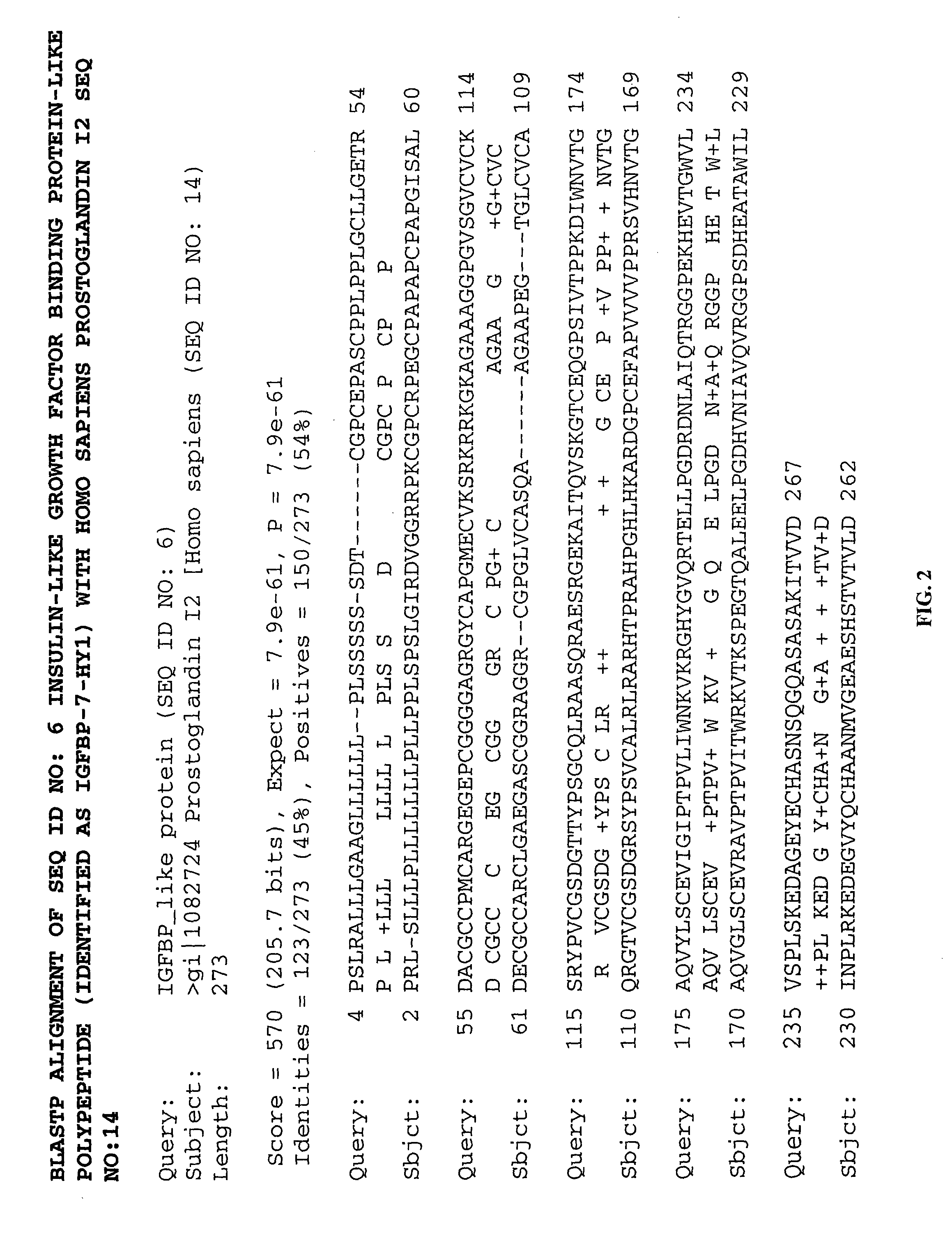
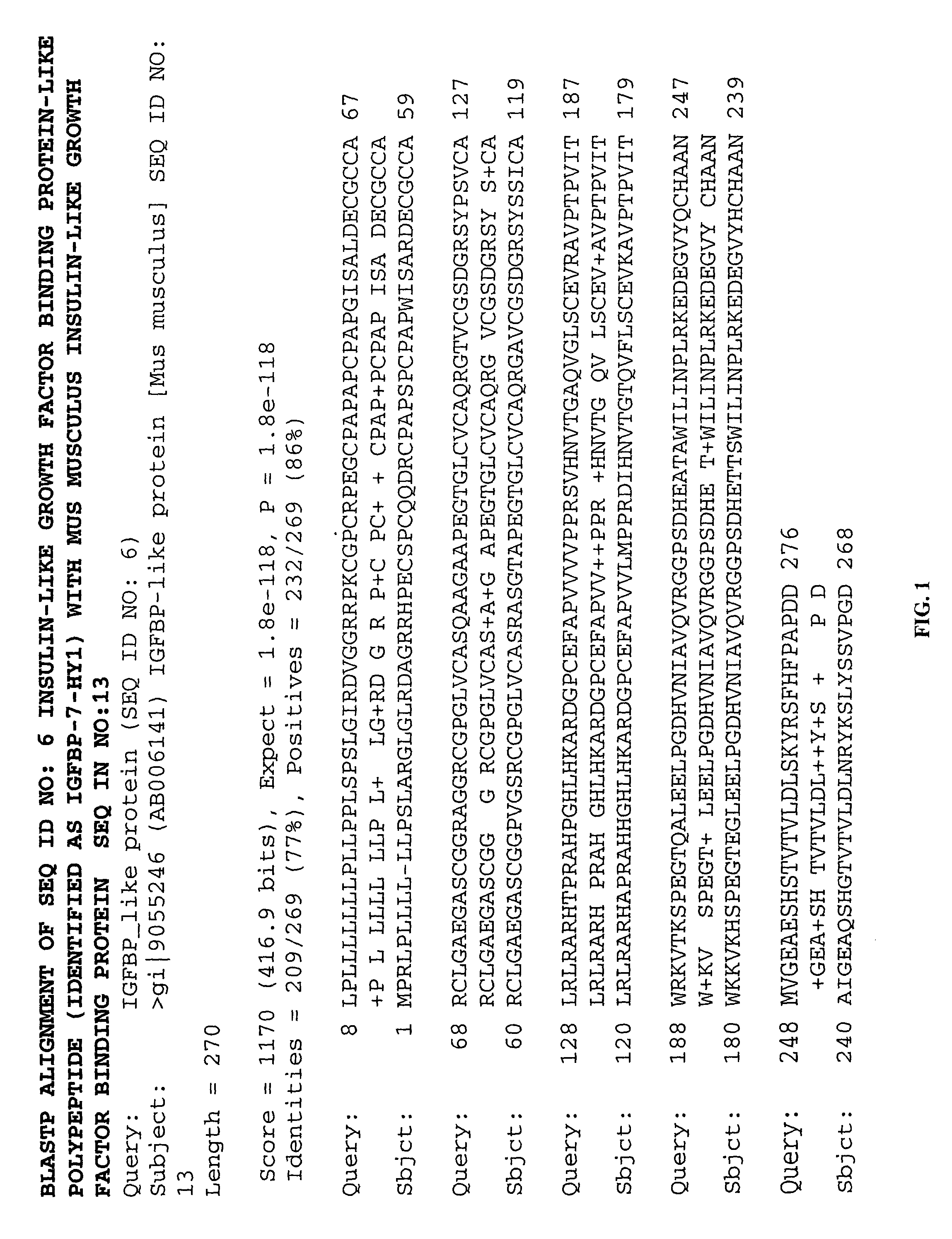
Methods of therapy and diagnosis using insulin-like growth factor binding protein-like polypeptides and polynucleotides
InactiveUS20060073514A1Promote wound healingReduced activityPeptide/protein ingredientsReceptors for hormonesNucleotideMutant
Owner:NUVELO INC
Pharmaceutical Proteins, Human Therapeutics, Human Serum Albumin Insulin, Native Cholera Toxin B Subunit on Transgenic Plastids
InactiveUS20110179530A1Eliminate needLarge biomassBryophytesMicroorganismsOral tolerizationInsulin-like growth factor
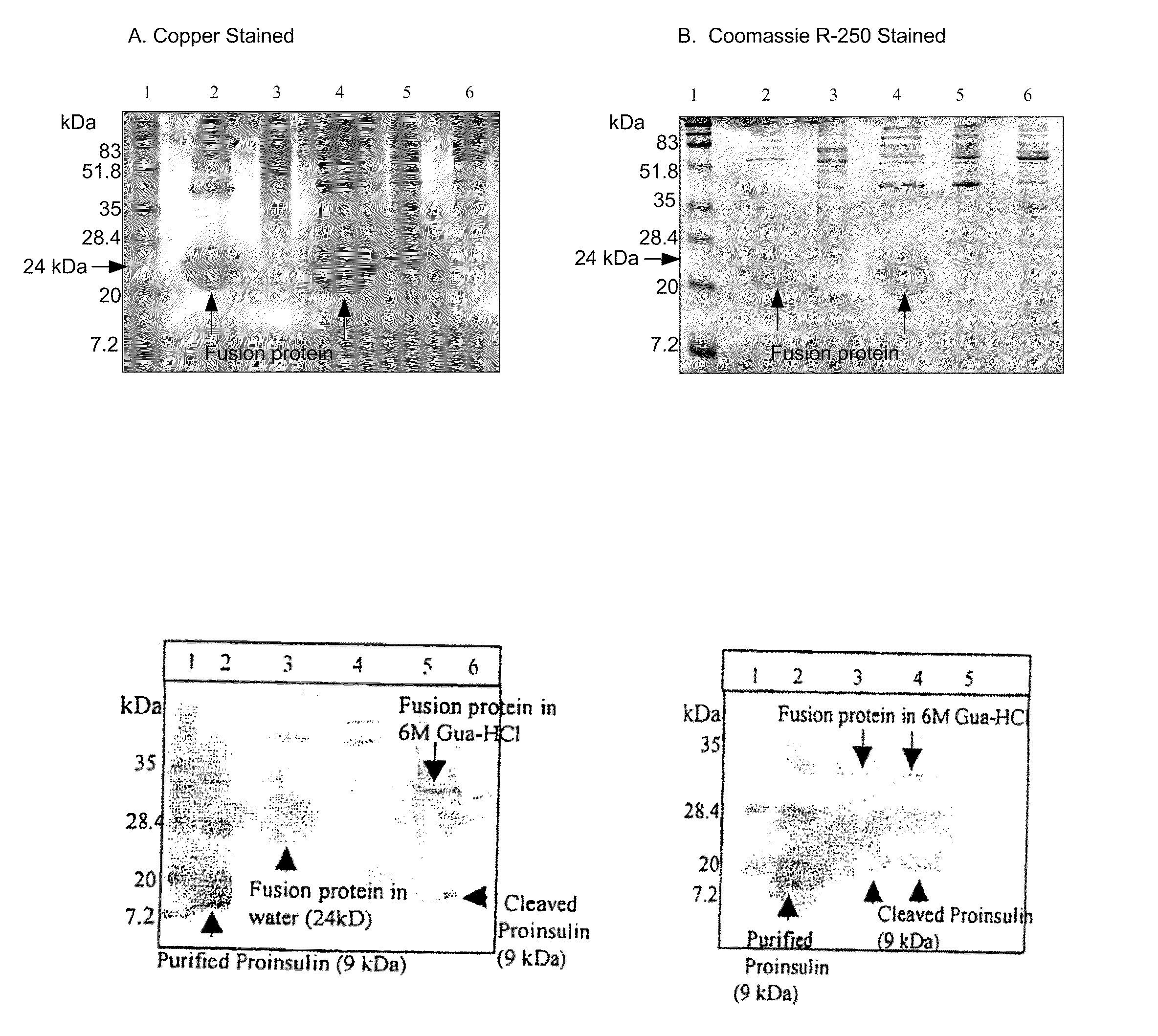
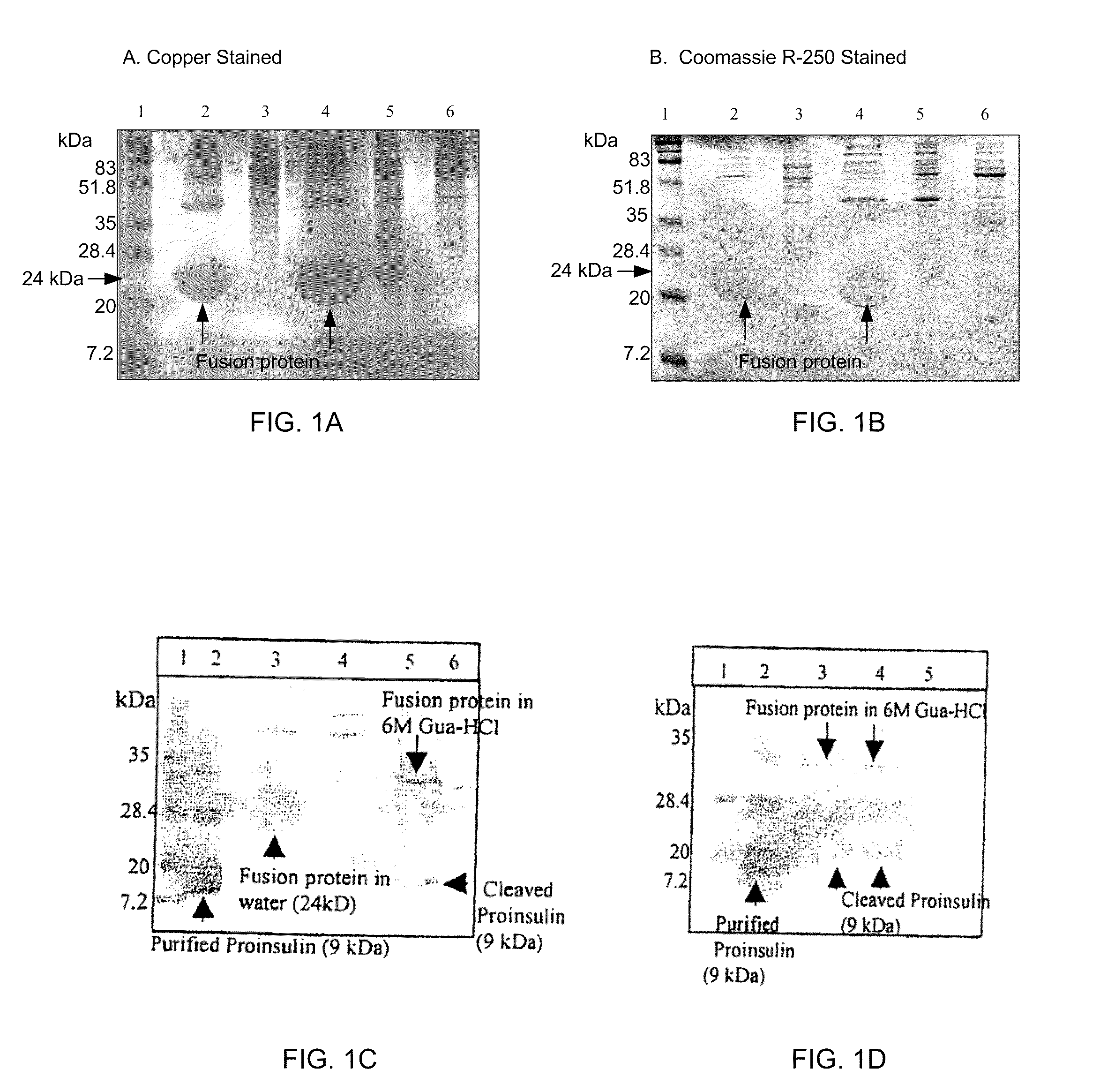
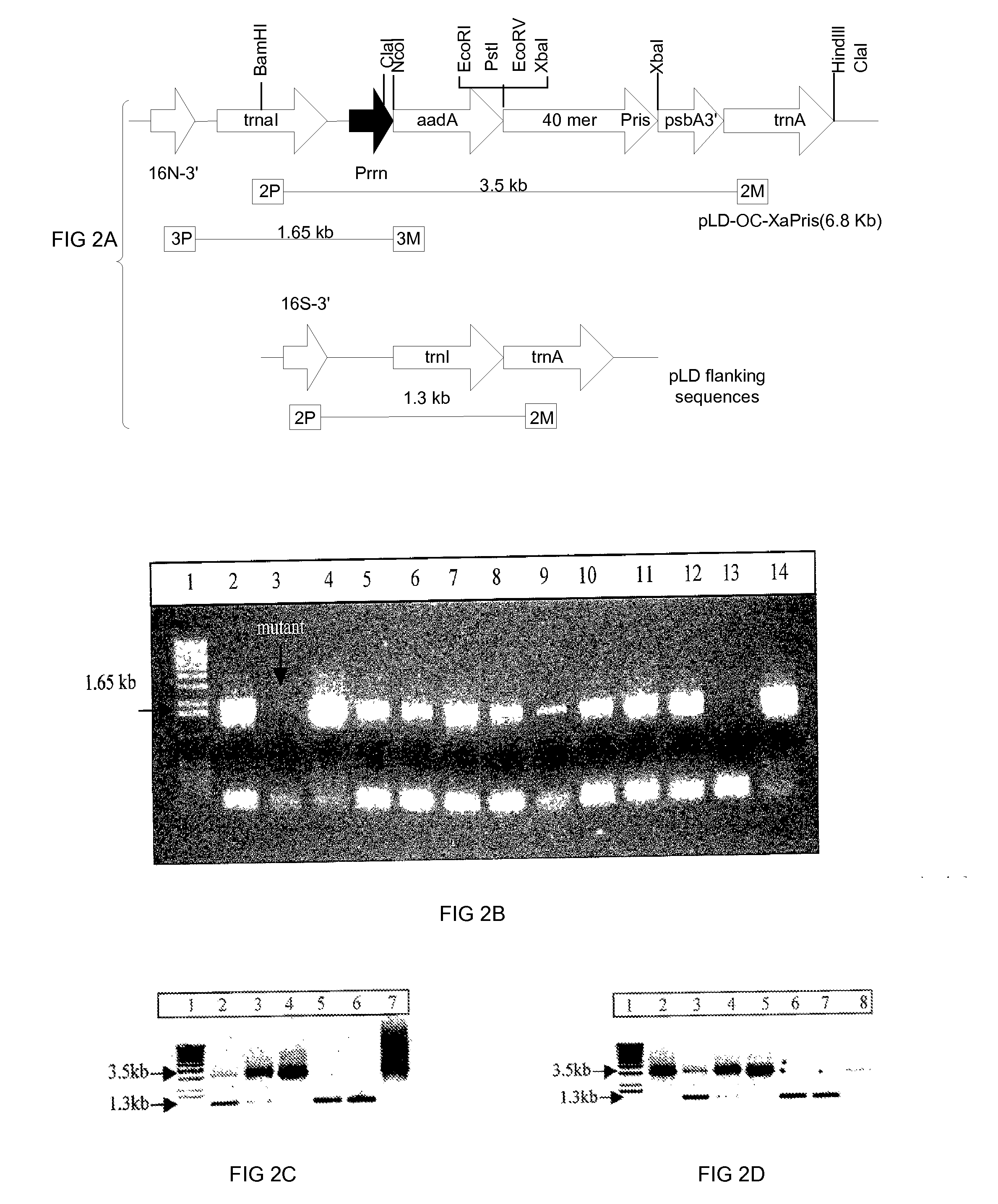
This invention relates in part to synthesizing high value pharmaceutical proteins in transgenic plants by chloroplast expression for pharmaceutical protein production. We use poly(GVGVP), for example, as a fusion protein to enable hyper-expression of insulin and to accomplish rapid one step purification of fusion peptides utilizing the inverse temperature transition properties of this polymer. We also use insulin-CTB fusion protein in chloroplasts of nicotine free edible tobacco (LAMD 605) for oral delivery. This invention includes expression of native cholera toxin B subunit gene as oligomers in transgenic tobacco chloroplasts which may be utilized in connection with large-scale production of purified CTB, as well as an edible vaccine if expressed in an edible plant, as a transmucosal carrier of peptides to which it is fused to enhance mucosal immunity, and / or to induce oral tolerance of the products of these peptides. The present invention also relates in part to recombinant DNA vectors for enhanced expression of human serum albumin, insulin-like growth factor I, and interferon-α 2 and 5, via chloroplast genomes.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
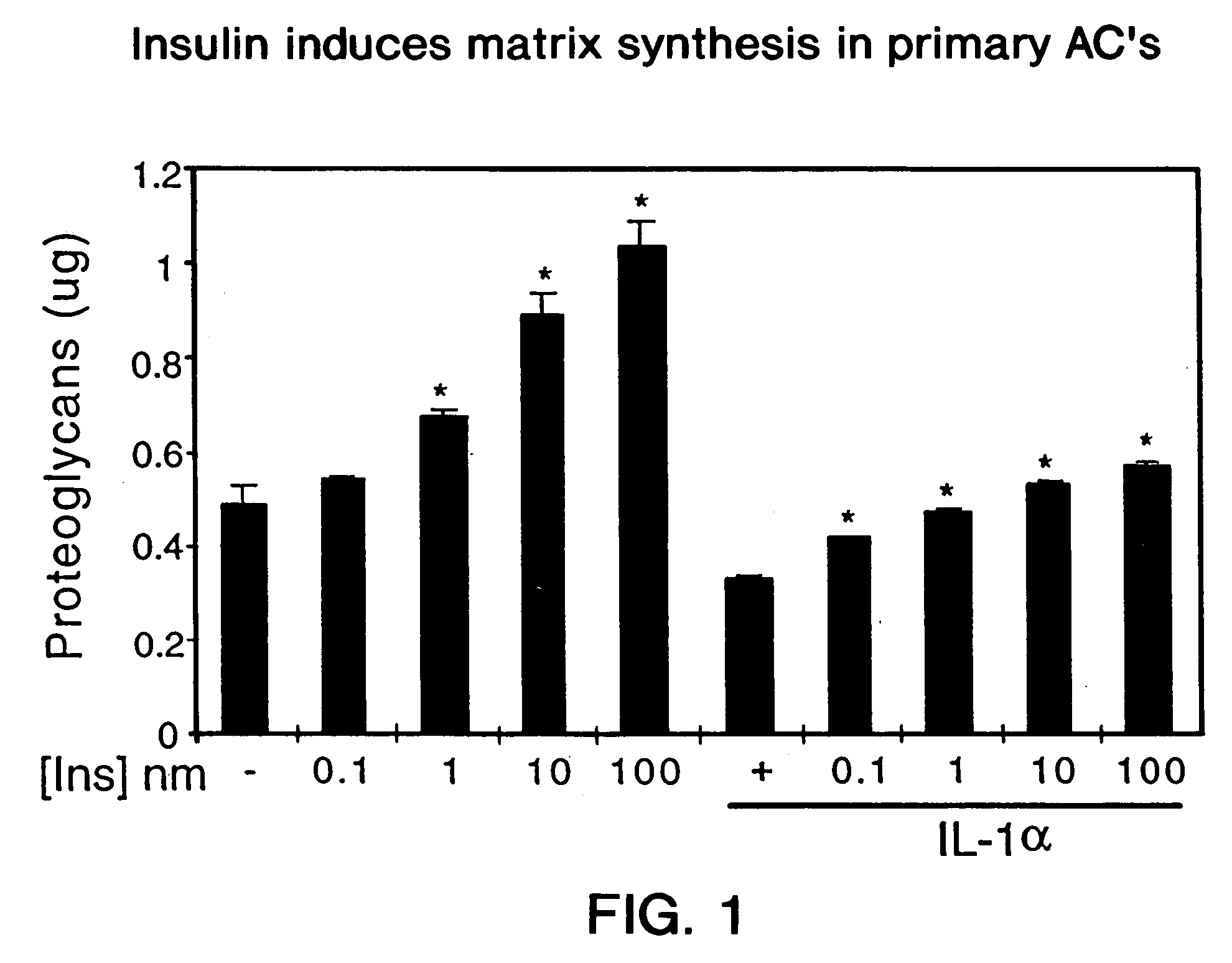
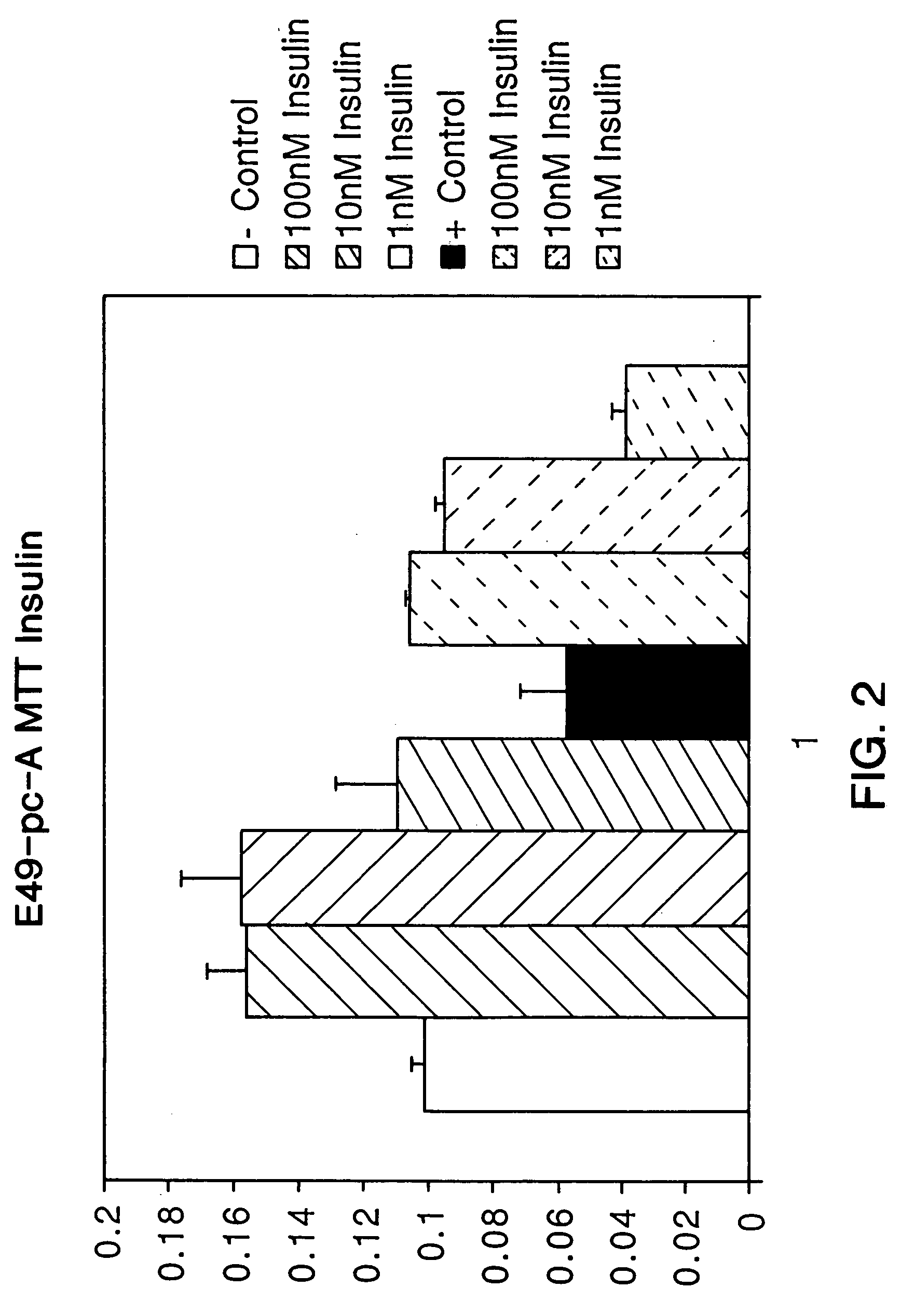
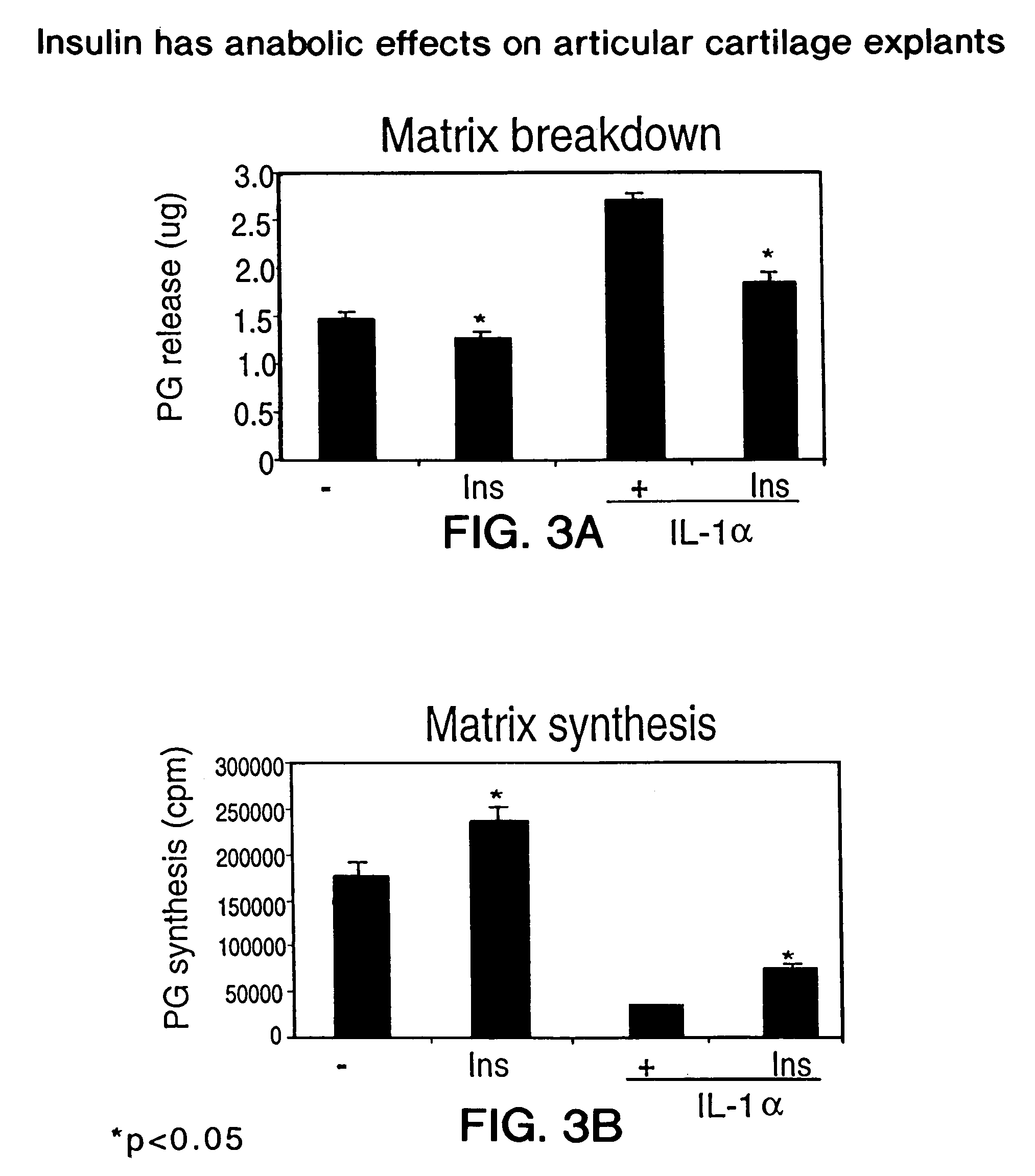
Use of insulin for the treatment of cartilaginous disorders
InactiveUS7268112B2Maintaining and enhancing and promoting growthEasy to degradeAntibacterial agentsSenses disorderDiseaseArthritis
Owner:GENENTECH INC
Compositions and methods for preserving insulin-producing cells and insulin production and treating diabetes
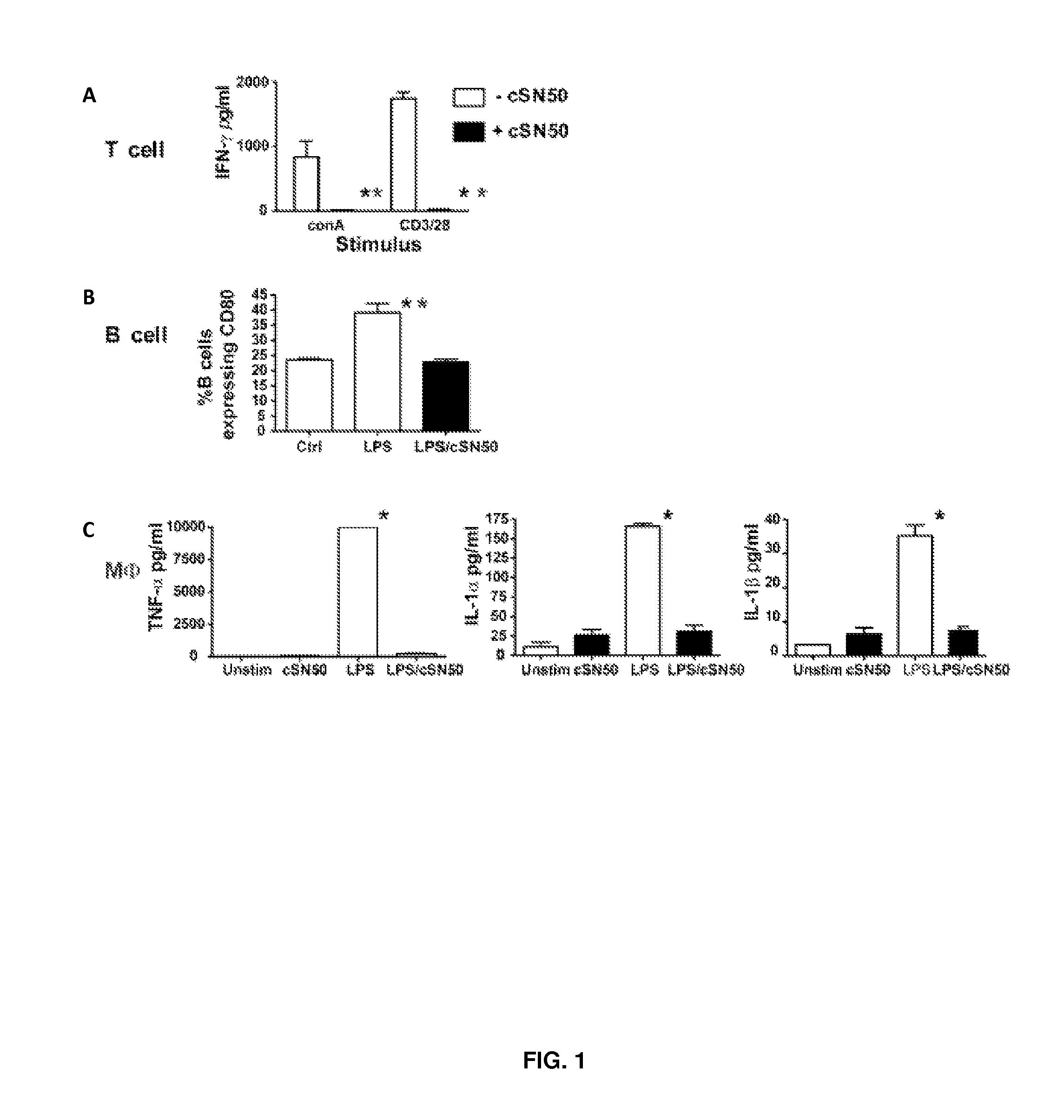
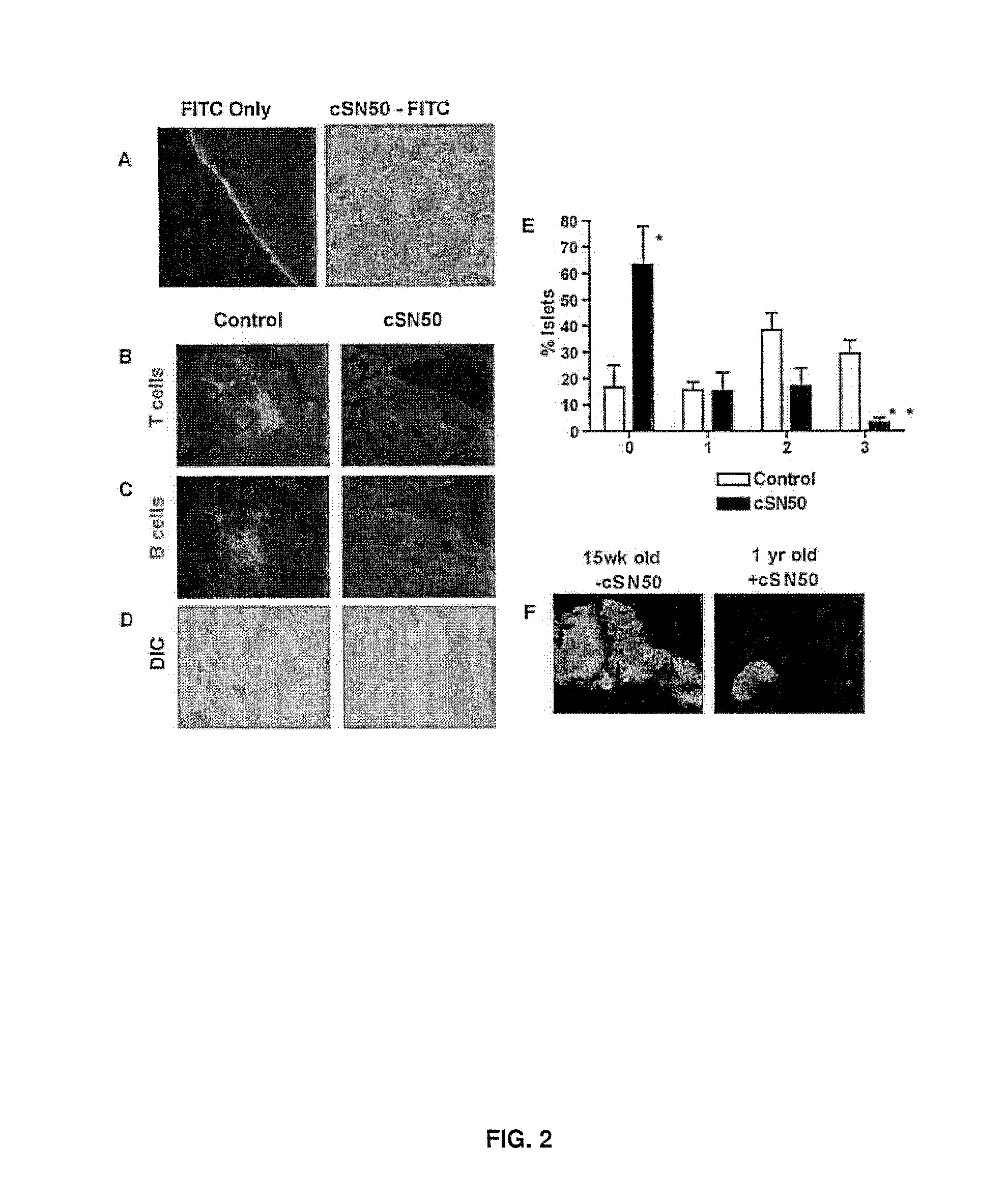
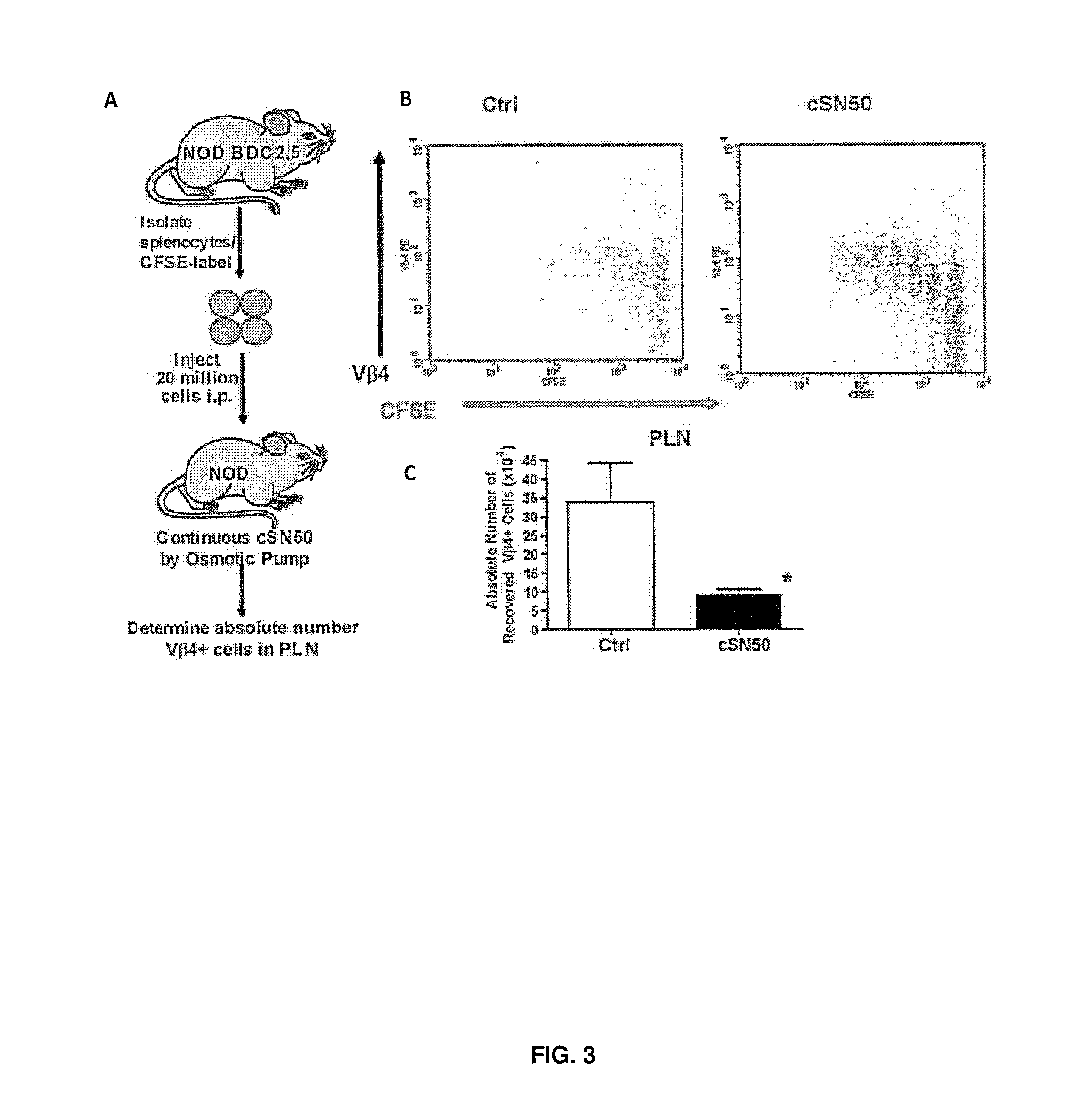
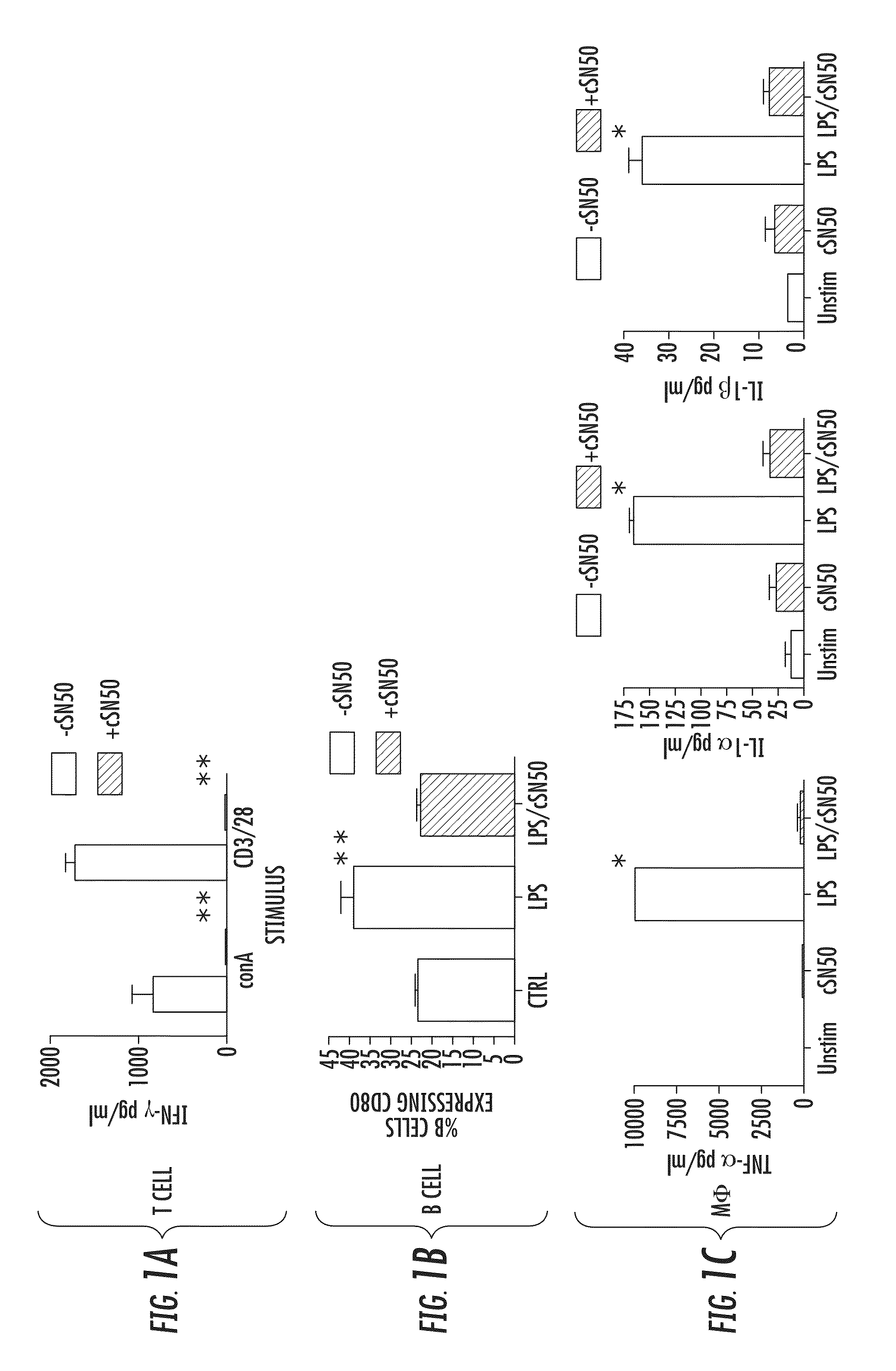
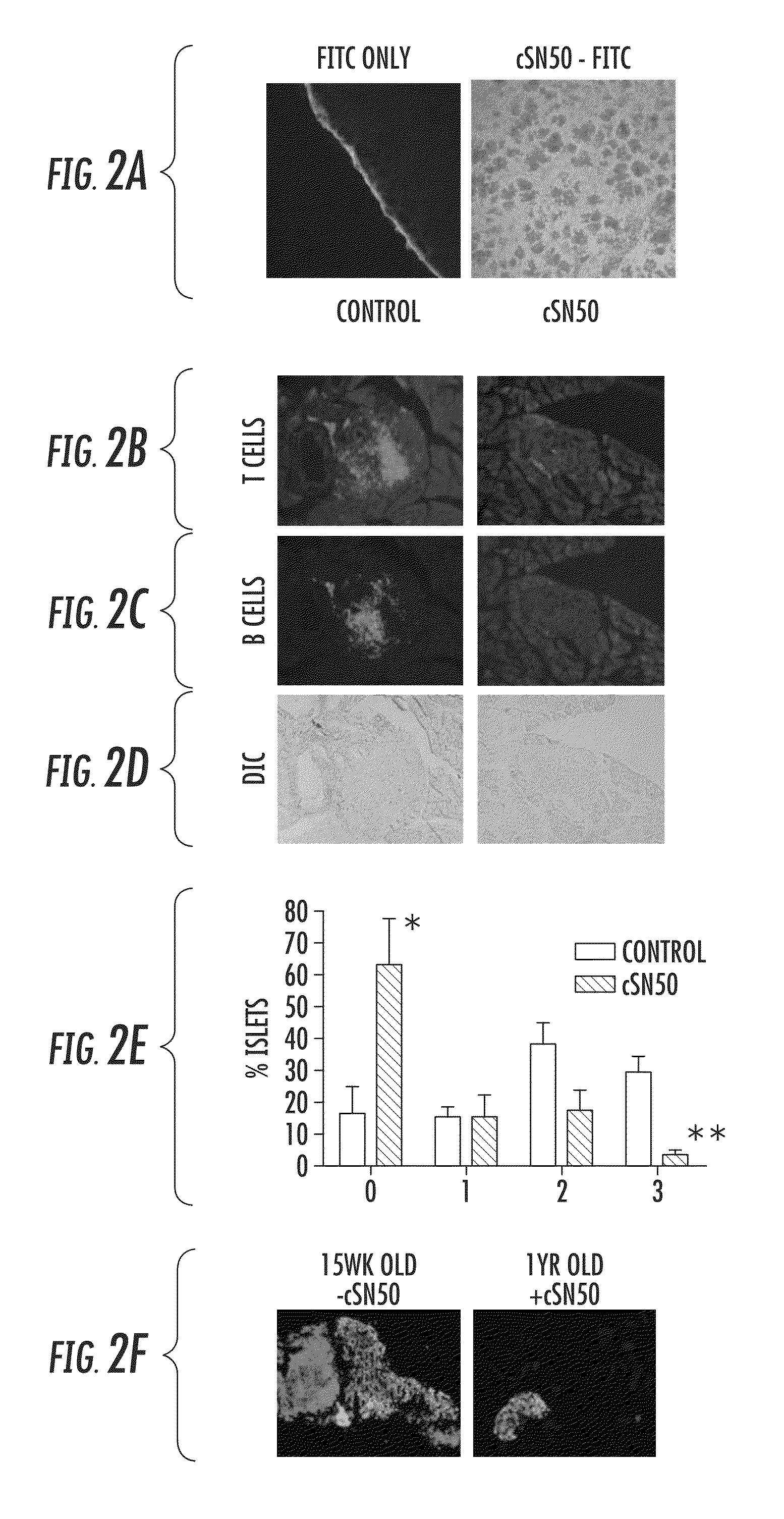
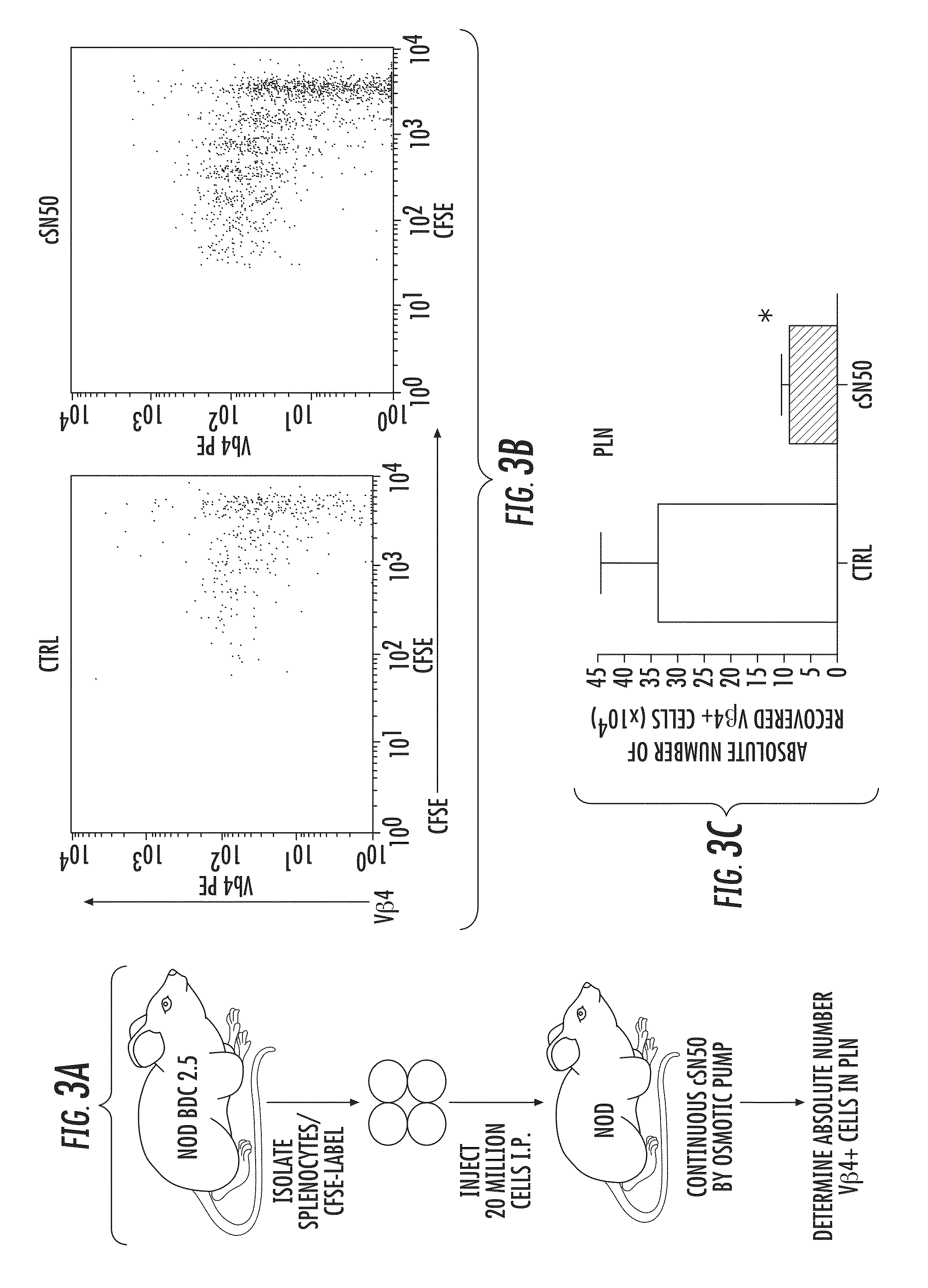
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of blood glucose levels and may reducing insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
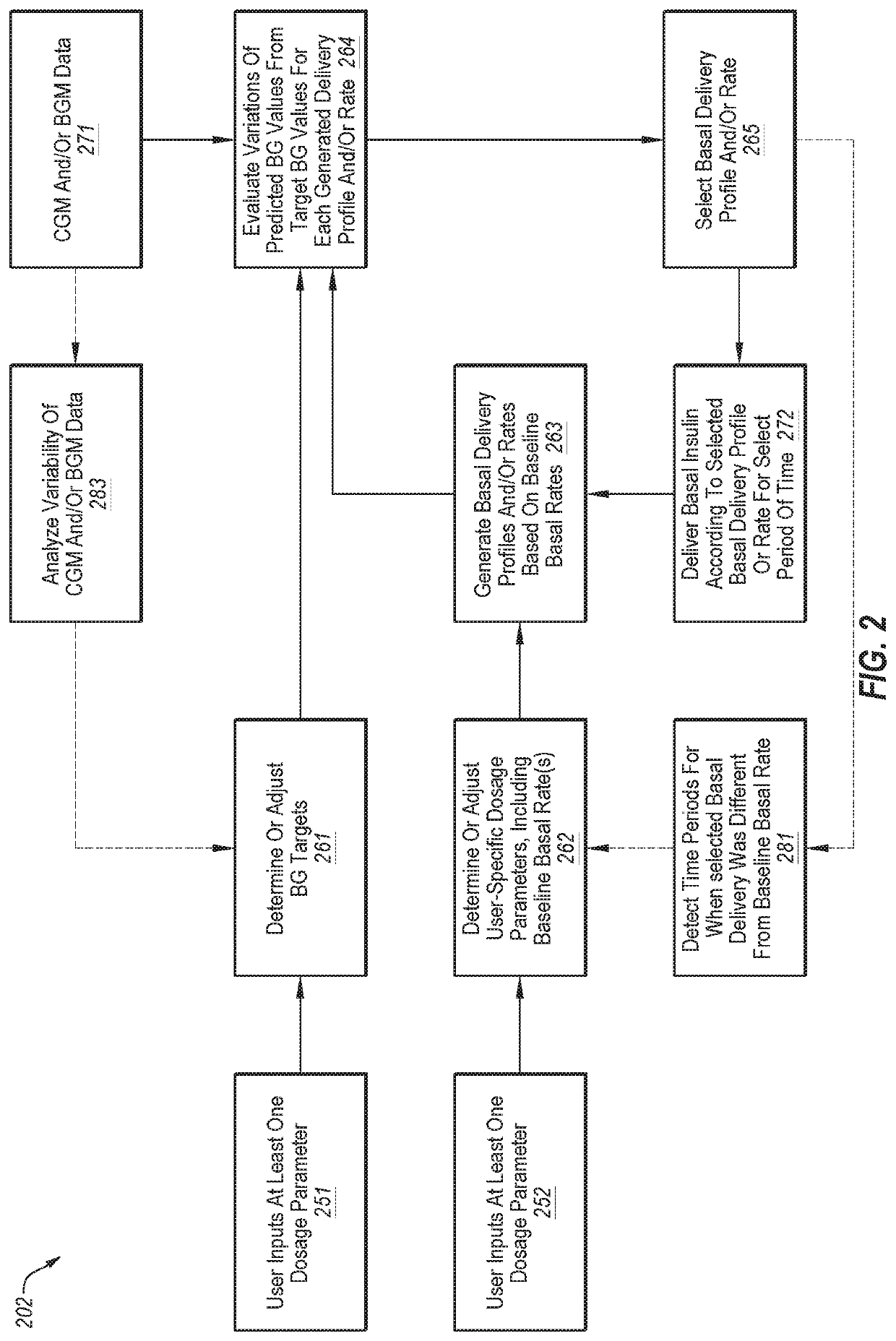
Adjusting insulin delivery rates
ActiveUS10806859B2Simple deliveryReduce cognitive loadHealth-index calculationDrug and medicationsPhysiologyHypoglycemia
A method may include displaying to a user an interface at which the user inputs a fear of hypoglycemia index (FHI), the FHI corresponding to an acceptable probability of a blood glucose level being below a threshold blood glucose level. The method may also include receiving blood glucose data for a person with diabetes (PWD). The method may additionally include calculating a probability of the PWD having a blood glucose level below the threshold blood glucose level based on the variability of the received blood glucose data. The method may also include setting one or more target blood glucose levels to align the probability of the PWD having a blood glucose level below the threshold blood glucose level with the acceptable probability associated with the user input FHI. The method may additionally include delivering insulin, using the insulin delivery device, based on the target blood glucose level.
Owner:INSULET CORP
System for treating heart disease and cardiovascular disease in diabetic and non-diabetic patients
InactiveUS20050123579A1Improving dietary fuelCorrect overutilizationPeptide/protein ingredientsFood preparationHeart diseaseFatty acid
The present invention is a system capable of improving the dietary fuel capabilities of diabetics and metabolically impaired patients and correct an overutilization of free fatty acids associated with heart and cardiovascular disease in diabetic and non-diabetic patients. The current invention is the treating of heart disease and cardiovascular disease using insulin pulses to a patient utilizing Chronic Intermittent Intravenous Insulin Therapy to achieve an increase dietary fuel capabilities and correct overutilization of free fatty acids associated with heart and cardiovascular disease in both diabetic and non-diabetic patients.
Owner:AOKI THOMAS T
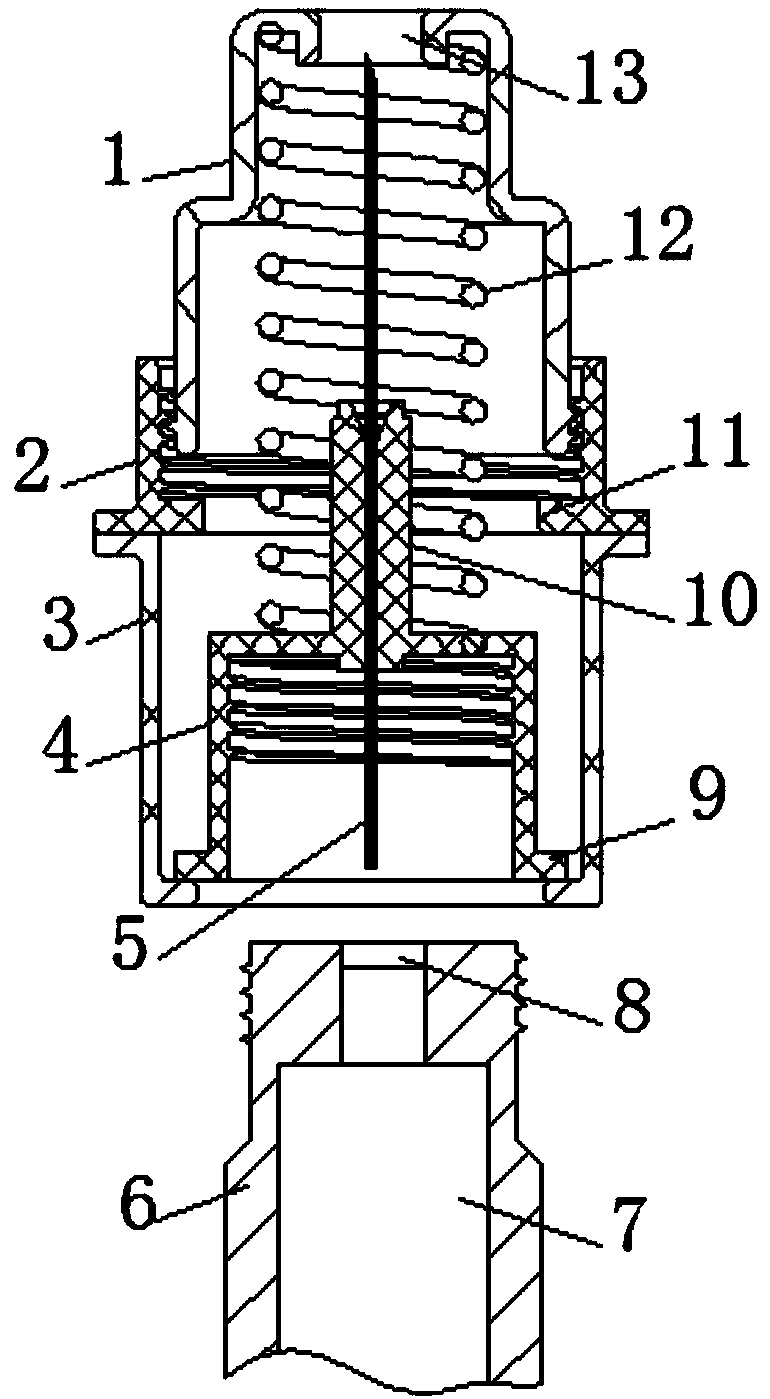
A rotary insulin pen needle
PendingCN109172955AAvoid stab woundsAvoid deformationInfusion needlesInsulin pen needleInsulin injection
A rotary insulin pen needle includes a needle shield housing, the top end of the needle protective shell body is welded with a needle protective cap fixing seat, and a needle rotating fixing seat is embedded in the needle protection outer shell body, the top end of the needle protective cap fixing seat is bolted with a protective cap on the needle, and the inner side of the bottom end of the needle protection cap fixing seat is provided with a rotating fixing seat baffle plate, the outer side of the insulin injection needle of the invention is provided with a needle protective outer shell, theinsulin injection needle is telescopic, When an insulin needle is needed, the needle can be protruded by pushing the needle to rotate the holder, At that end of use, the insulin injection needle canbe shrink into the needle protective housing to prevent the needle from bee damaged and deformed, and the needle protective housing is of a combined structure, which is more conducive to the recoveryand reuse of the recover needle, meanwhile, the insulin injection needle after use can be prevented from being pricked to the hand when being treated, and is safer to be treated.
Owner:ANHUI HONGYU WUZHOU MEDICAL DEVICES CO LTD
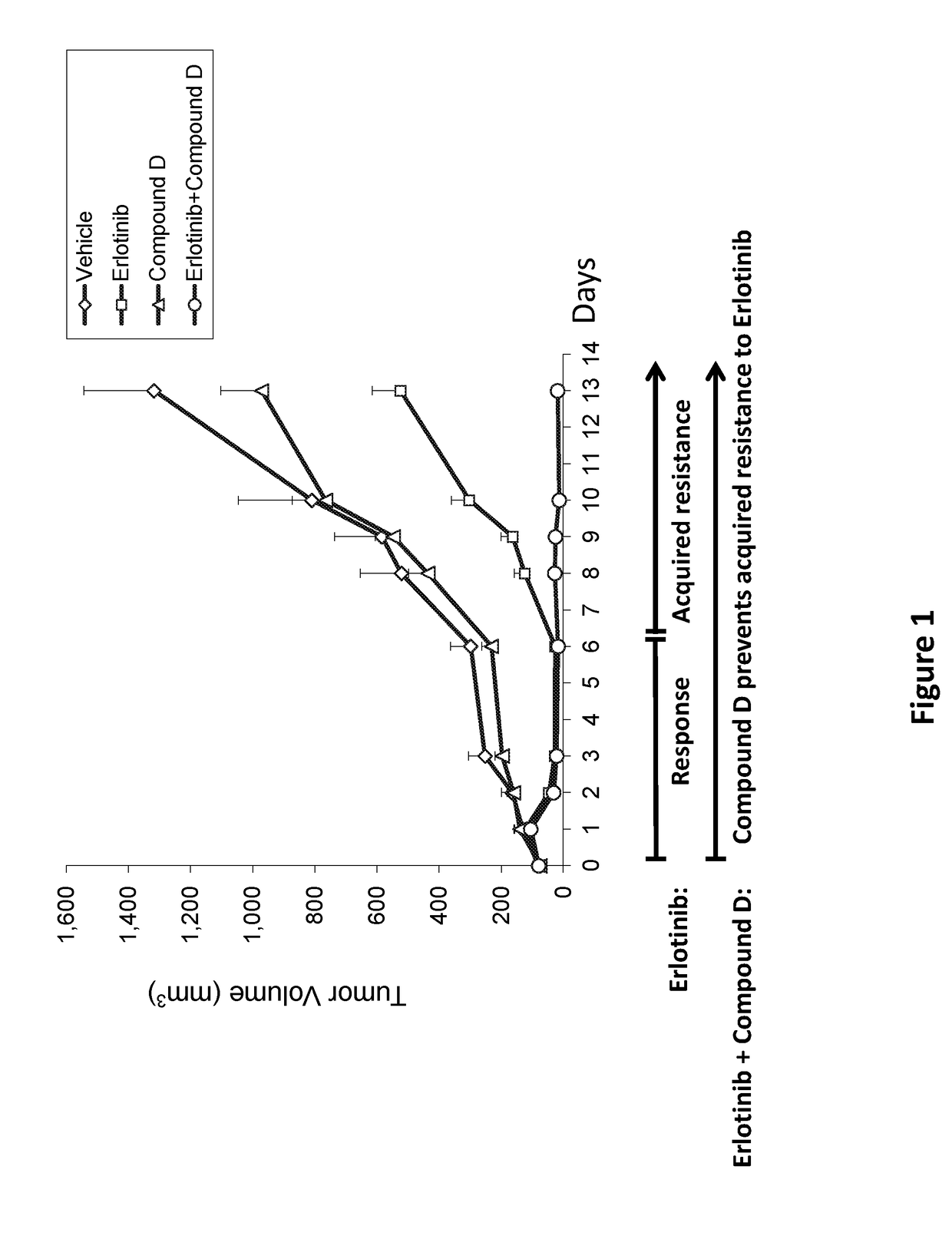
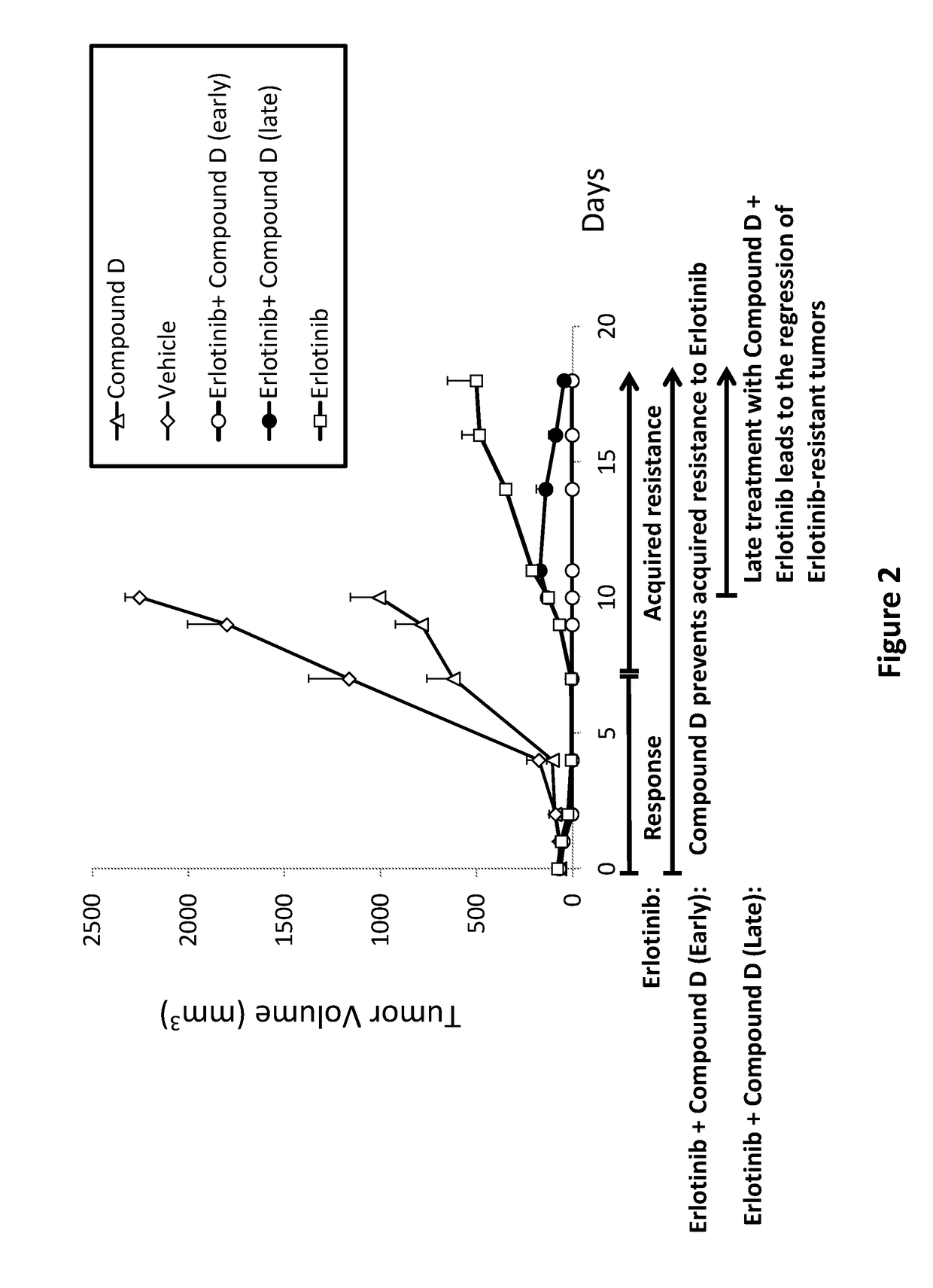
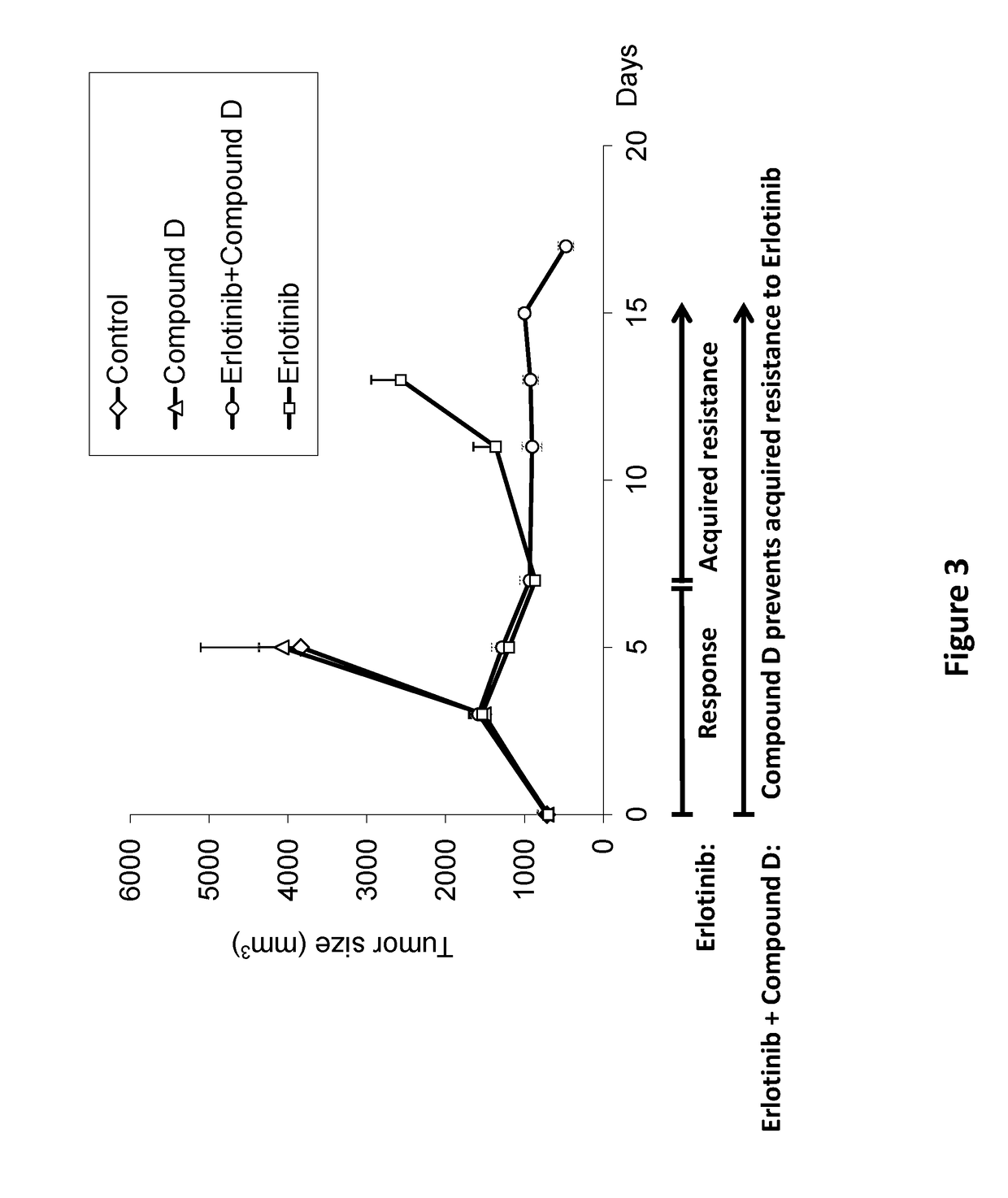
Combinations of irs/stat3 dual modulators and Anti-cancer agents for treating cancer
ActiveUS20180028475A1Preventing and delaying tumor recurrenceAvoid resistanceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAcquired resistanceImmunotherapeutic agent
Provided is a treatment of cancer using a dual modulator of Insulin Receptor Substrate (IRS) and signal transducer and activator of transcription 3 (Stat3), as well with other agents. The combination can be used to treat a tumor that has developed resistance to an EGFR inhibitor, EGFR antibody, mTOR inhibitor, MEK inhibitor, mutated B-Raf inhibitor, chemotherapeutic agents, and certain combinations thereof, or to prevent acquired resistance of a tumor to any of said inhibitors or agents, or to prevent tumor recurrence following cease of treatment with any of said inhibitors or agents or a combination thereof. The subject matter further provides a treatment of cancer using combination therapy comprising a dual modulator of IRS and Stat3, in combination with an immunotherapy agent. The combination can be used to sensitize a tumor to immunotherapy.
Owner:TYRNOVO LTD
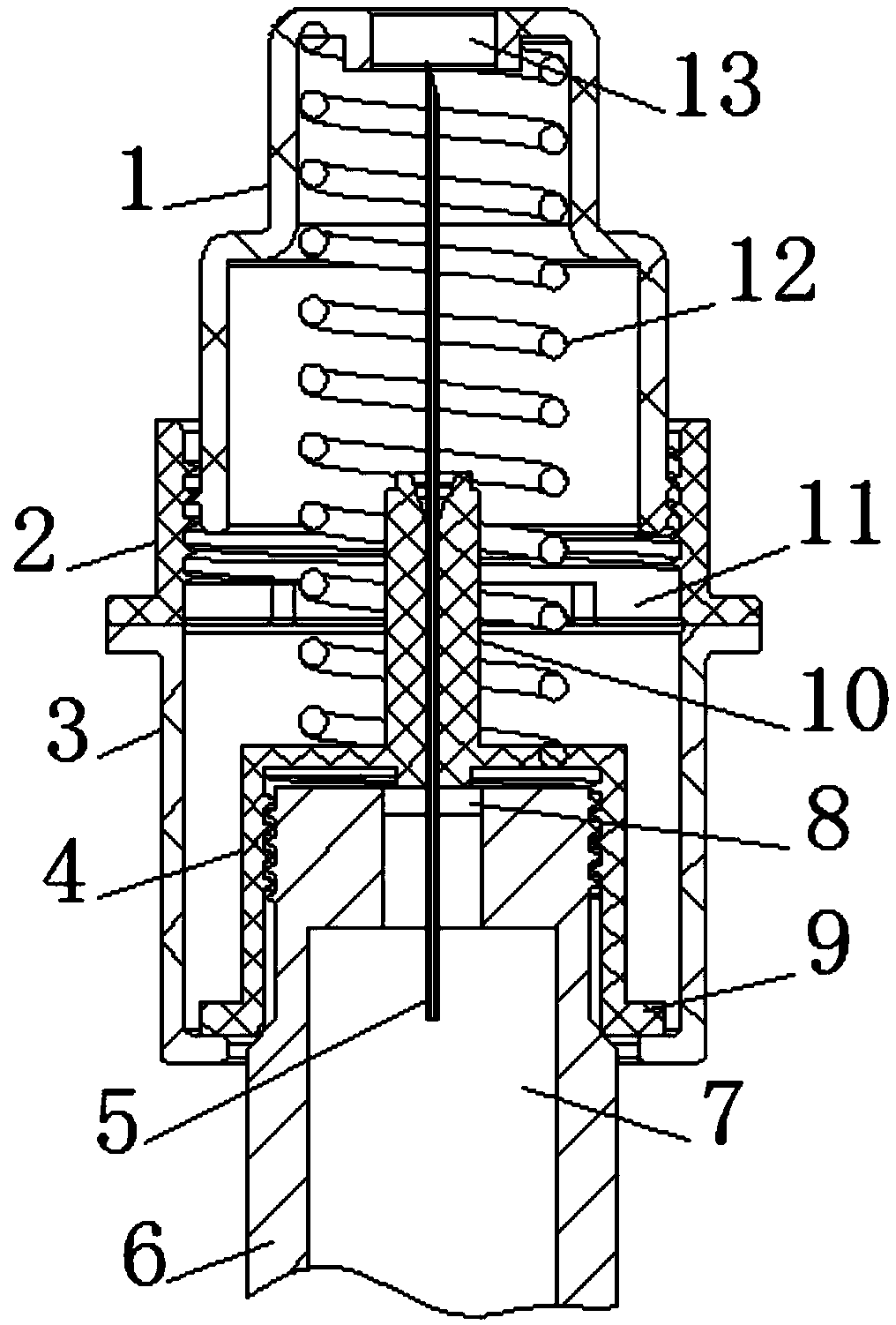
Matched needle for safety insulin pen
ActiveCN105944190AAvoid stabbingReduce the risk of cross-infectionMedical devicesInfusion needlesSyringe needleScrew thread
The invention relates to a matched needle for a safety insulin pen. The matched needle comprises a needle seat fixedly provided with a needle tube and a safety protection sleeve. The needle seat comprises a pen cap part in threaded fit with the end of the insulin pen and a sliding joint part coaxially and fixedly arranged at the front end of the pen cap part. The sliding joint part is cylindrical, the front end of the needle tube stretches out of the end of the sliding joint part, the safety protection sleeve is a bucket-shaped component, the inner diameter of the safety protection sleeve is in clearance fit with the sliding joint part, and the rear end of the safety protection sleeve is open. A needle hole allowing a needle tip to penetrate out is formed in the position, corresponding to the needle tube, of the front end of the safety protection sleeve. According to the matched needle for the safety insulin pen, the safety protection sleeve is arranged, after the matched needle for the insulin pen is used, the safety protection sleeve slides out forwards to cover the exposed needle tip, therefore, puncture is avoided, and the risk of cross infection is reduced.
Owner:ZHEJIANG KINDLY MEDICAL DEVICES
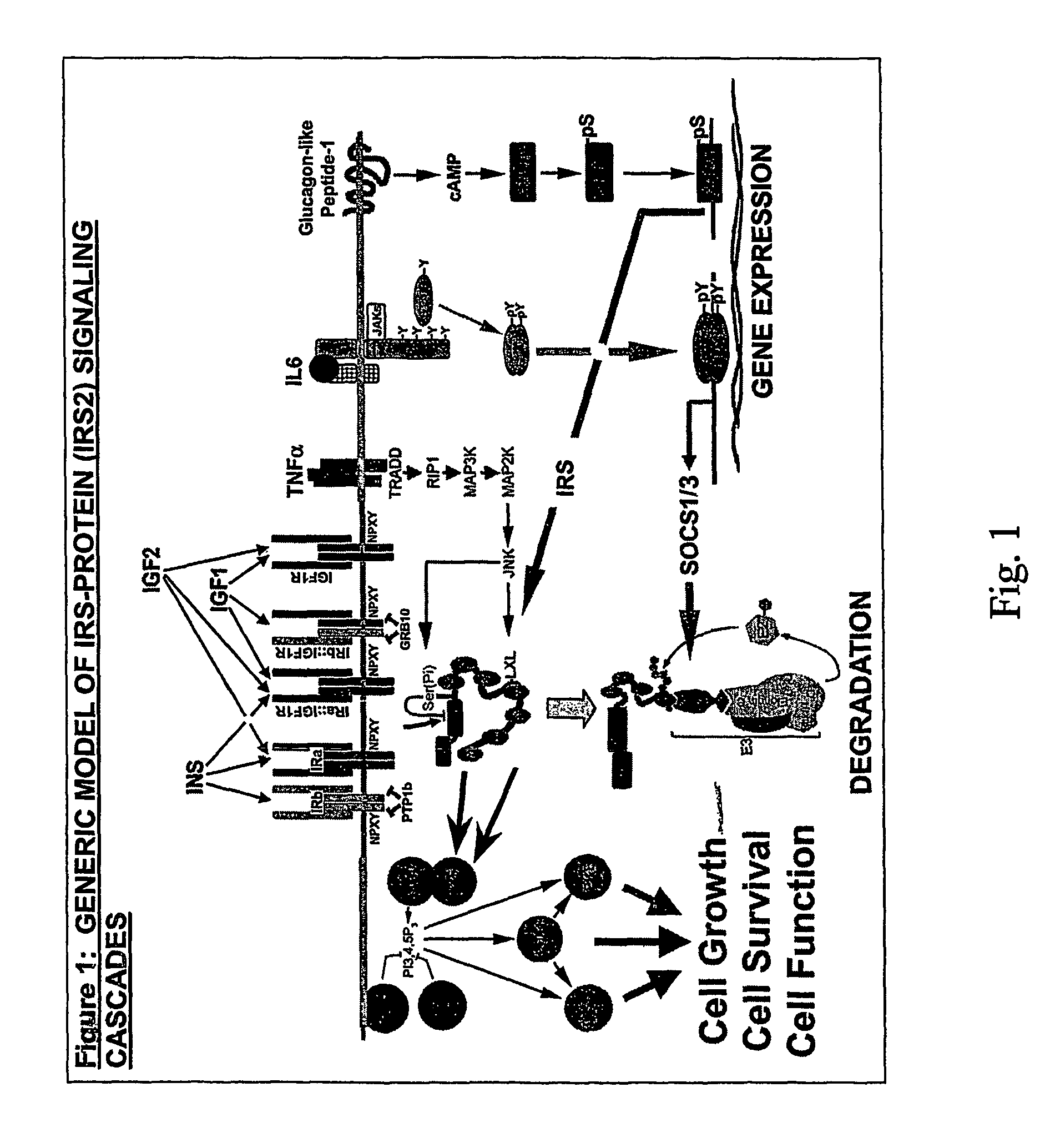
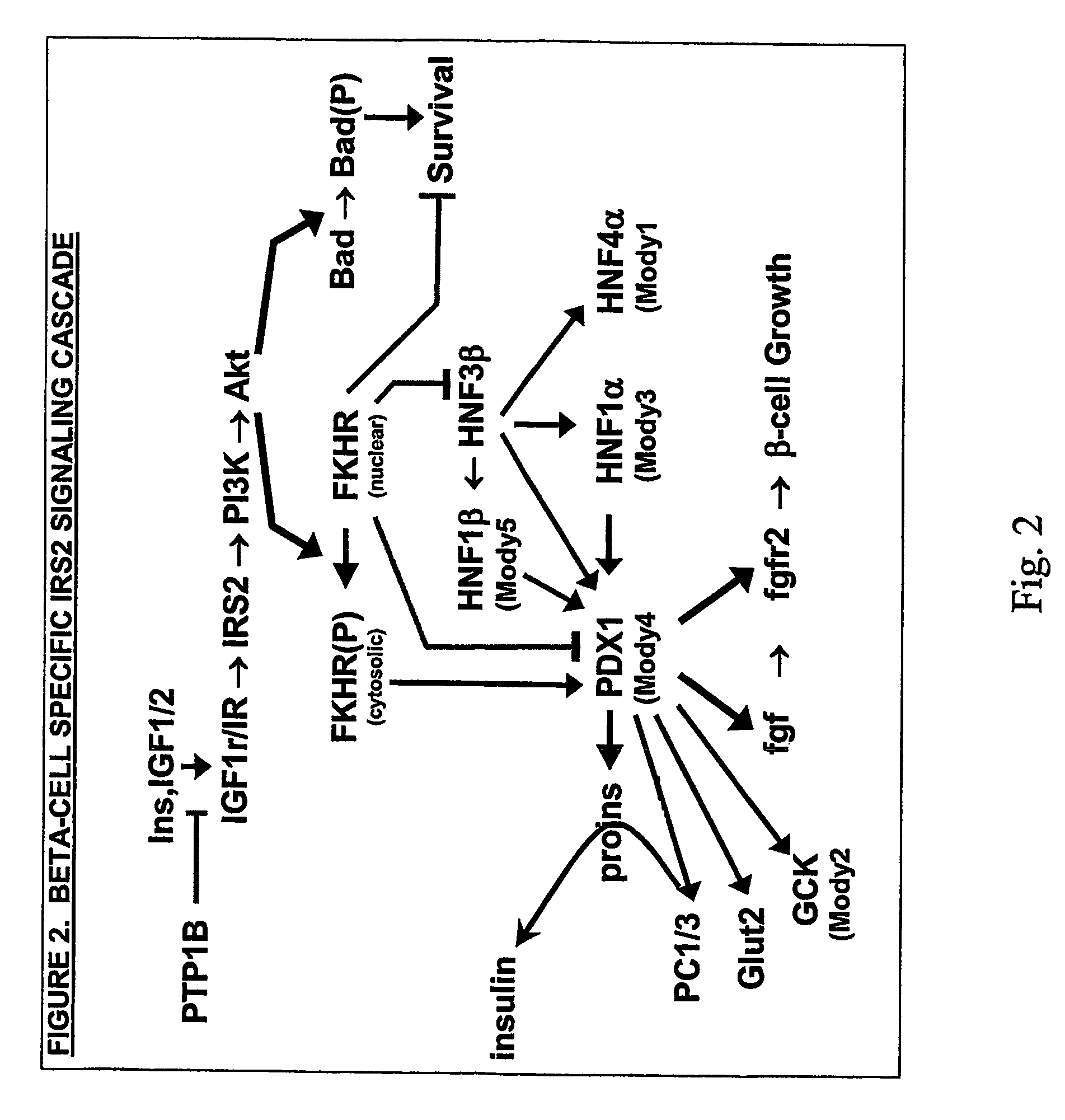
Irs modulators
ActiveUS20060241030A1Less amount of bindingImprove expression levelCompound screeningNervous disorderInsulin-like growth factorFunctional activity
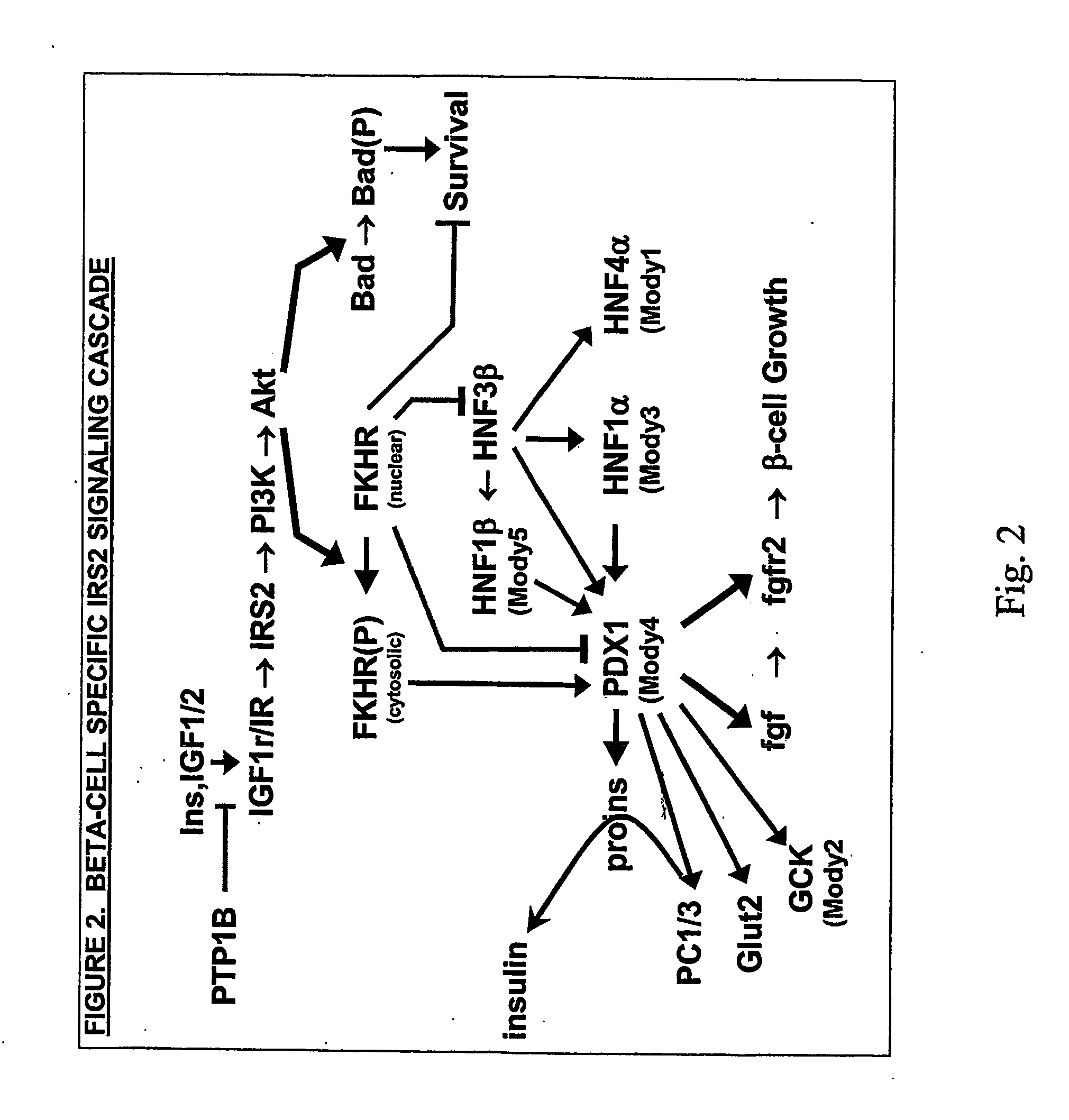
This invention is directed to a general method for the chronic treatment, potential cure, or prevention of various metabolic and related diseases in people, including diabetes, by modulating IRS2 activity in cells and tissues in the body. IRS1 and IRS2 are part of the insulin or insulin-like growth factor signaling pathway. By upregulating the levels or functional activity of IRS2, insuling is used more efficiently by the body to control nutrient levels. By upregulating IRS2 levels or functional activity in pancreatic β-cells, glucose sensing and insulin secretion are enhanced.
Owner:HMI MEDICAL INNOVATIONS
Compositions for preserving insulin-producing cells and insulin production and treating diabetes
ActiveUS9388224B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsAutoimmune diabetesIn vivo
Nuclear Transport Modifiers such as cSN50 and cSN50.1, afford in vivo islet protection following a 2-day course of intense treatment in autoimmune diabetes-prone, non-obese diabetic (NOD) mice, a widely used model of Type 1 diabetes (T1D), which resulted in a diabetes-free state for one year without apparent toxicity and the need to use insulin. cSN50 precipitously reduces the accumulation of islet-destructive autoreactive lymphocytes while enhancing activation-induced cell death of T and B lymphocytes derived from NOD mice. cSN50 attenuated pro-inflammatory cytokine and chemokine production in immune cells in this model of human T1D. cSN50 also provides cytoprotection of beta cells, therefore preserving residual insulin-producing capacity. Because intracellular delivery of a Nuclear Transport Modifier peptide such as cSN50 and cSN50.1 can result in lowering of fasting blood glucose levels and may ameliorate (e.g., reduce, eliminate) insulin resistance, the compositions, methods and cells described herein can also be used for treating Type 2 diabetes (T2D).
Owner:VANDERBILT UNIV
Medicine composition containing insulin intensifier and miglitol
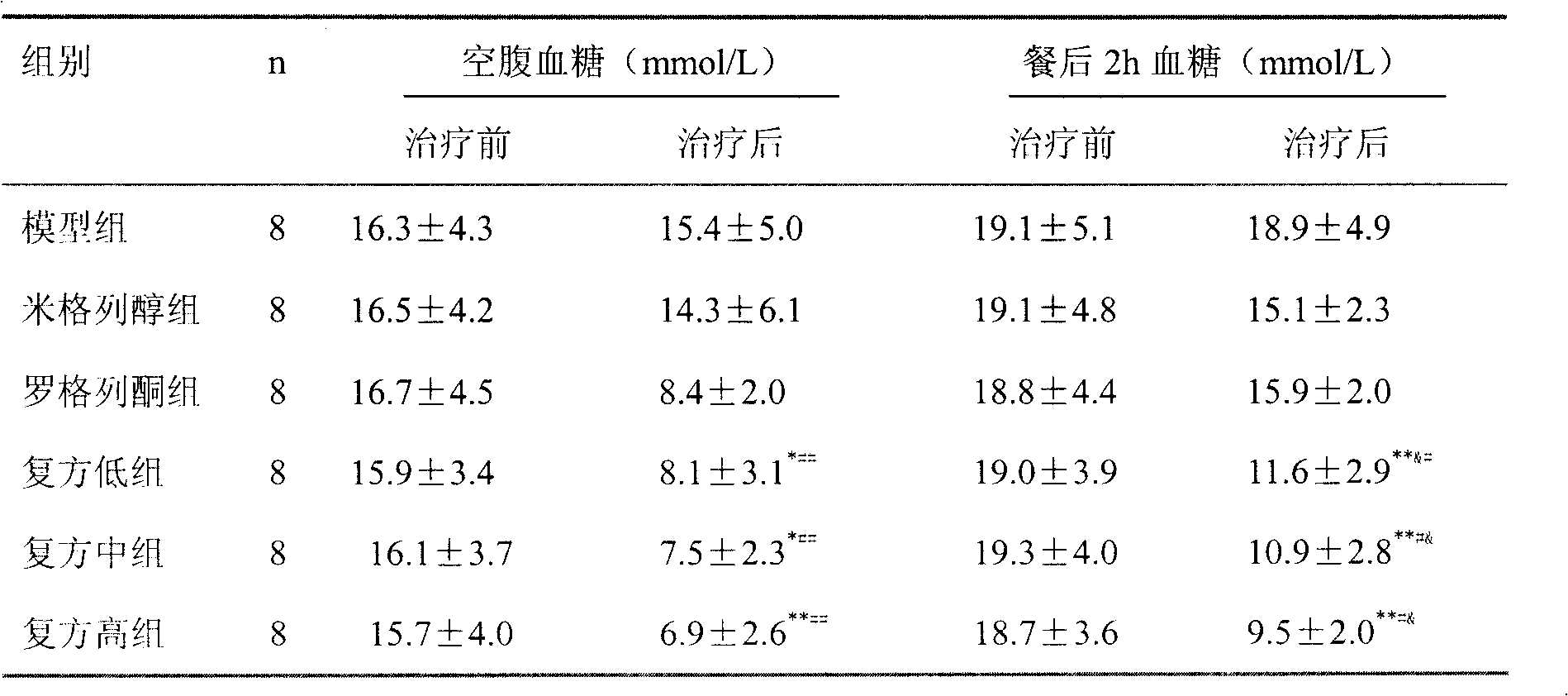
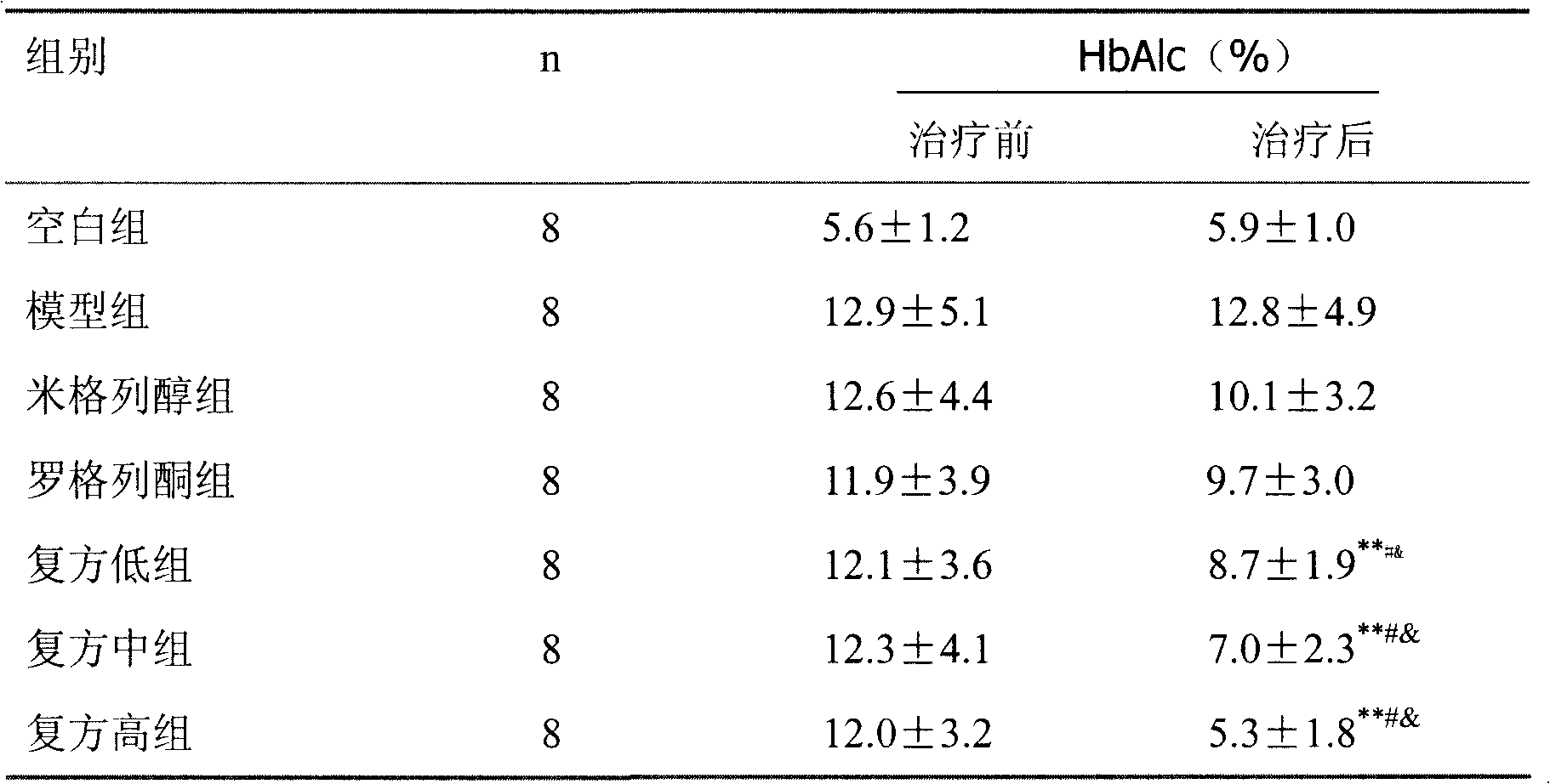
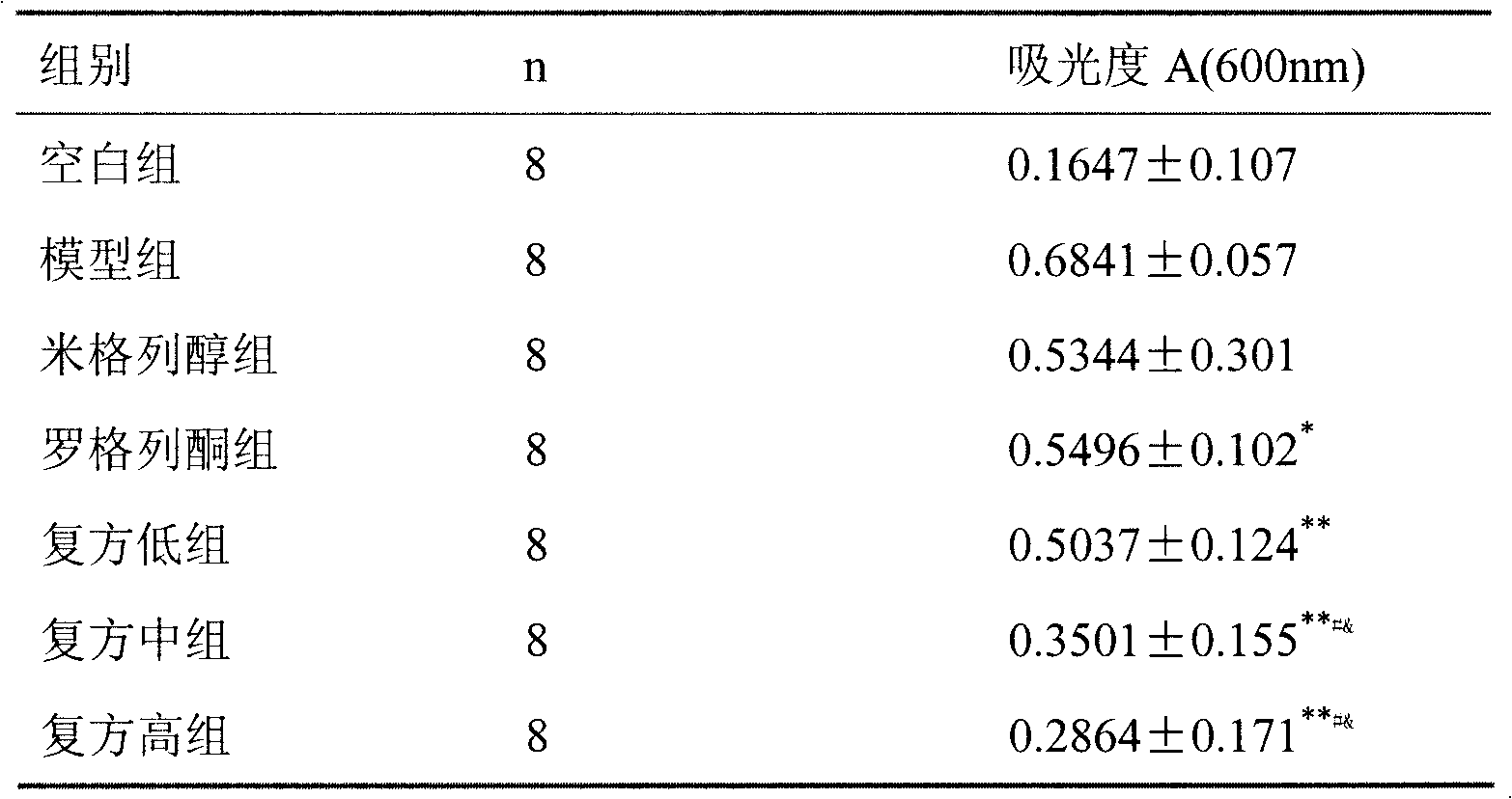
ActiveCN101121004BReduce complicationsImprove risk factorsMetabolism disorderPharmaceutical non-active ingredientsMiglitolAcute hyperglycaemia
The present invention relates to an oral blood-sugar-reducing compound medicinal preparation, which consists of an insulin sensitizer, a miglitol and auxiliary materials. Compared with the prior art, the present invention is characterized in that under a circumstance of a same curative effect, a separate dosage of the insulin sensitizer or the miglitol is reduced; at the same time compared with other hypoglycemic drugs, a side effect of the present invention is reduced; the insulin sensitizer and the miglitol have a synergistic effect: the insulin sensitizer and the miglitol respectively takecurative actions towards a patient with hyperglycemia synchronously according to the different pharmacological effects and directly provide the patient or a doctor with a scientific combined medication to improve the curative effect and provide a clinic or the patient with convenience.
Owner:LUNAN PHARMA GROUP CORPORATION
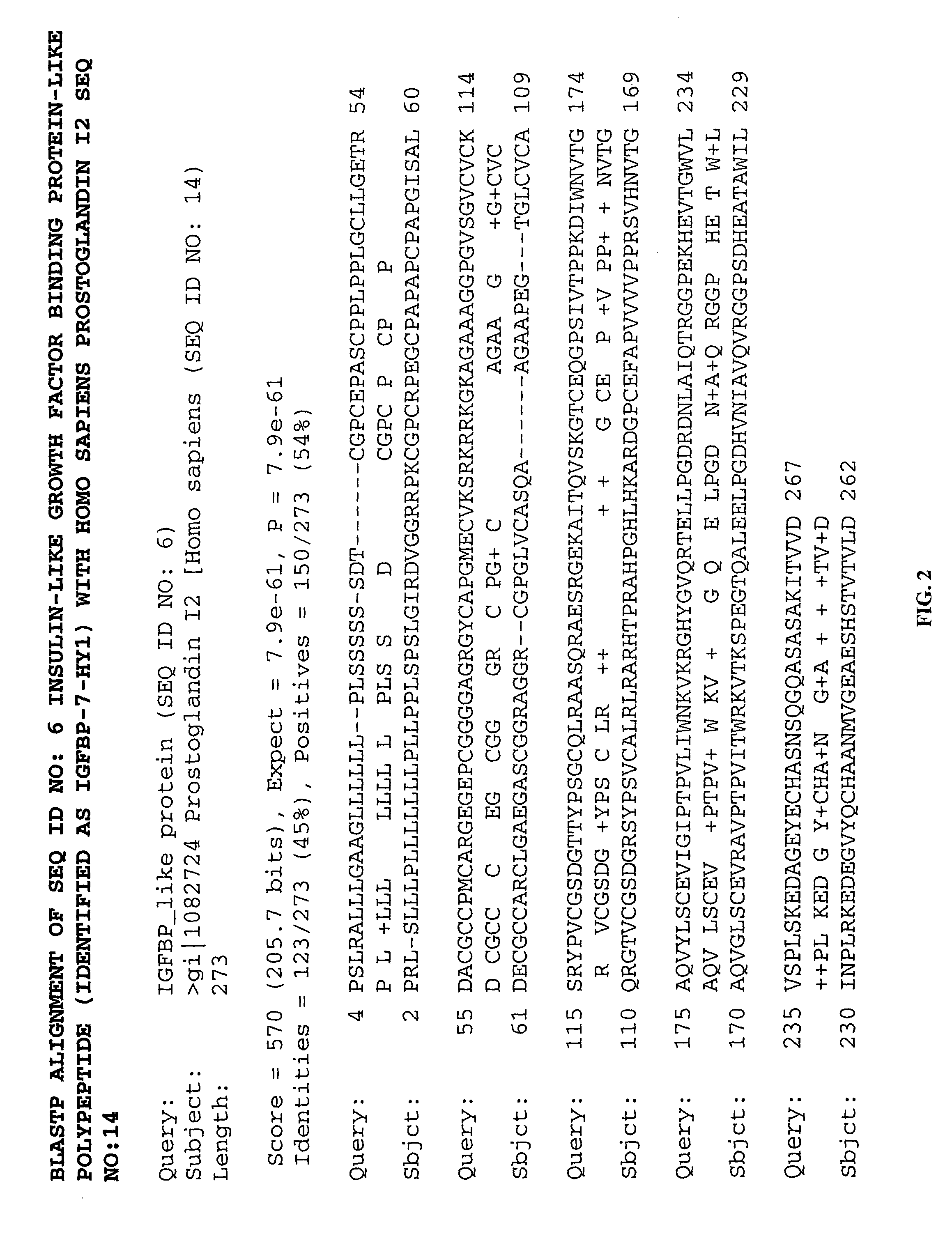
Methods of therapy and diagnosis using insulin-like growth factor binding protein-like polypeptides and polynucleotides
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to novel human secreted IGFBP-like polypeptides. Other aspects of the invention include vectors containing processes for producing novel human secreted IGFBP-like polypeptides, compositions, and methods of use for such polypeptides including in cancer.
Owner:NUVELO INC
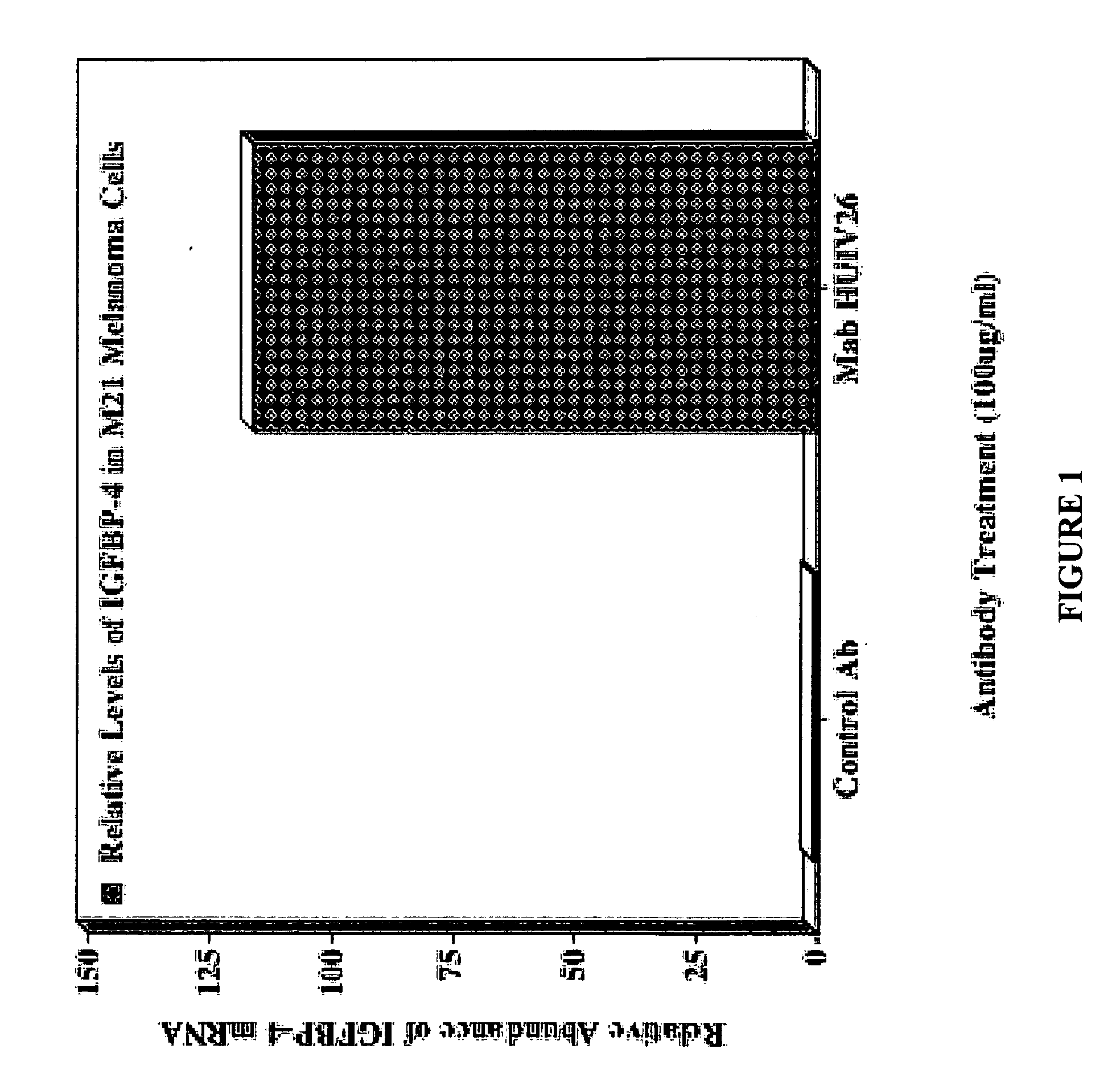
Inhibition of angiogenesis and tumor development by IGFBP-4
InactiveUS20060216237A1In-vivo radioactive preparationsAntibody ingredientsAbnormal tissue growthAngiogenesis growth factor
The invention provides compositions comprising IGFBP-4 and methods for inhibiting angiogenesis and tumor development processes, and for treating angiogenesis-dependent conditions, using an insulin growth factor binding protein, IGFBP-4.
Owner:NEW YORK UNIV
Method of screening activators and/or inhibitors of insulin receptor substrate 2
Owner:HMI MEDICAL INNOVATIONS
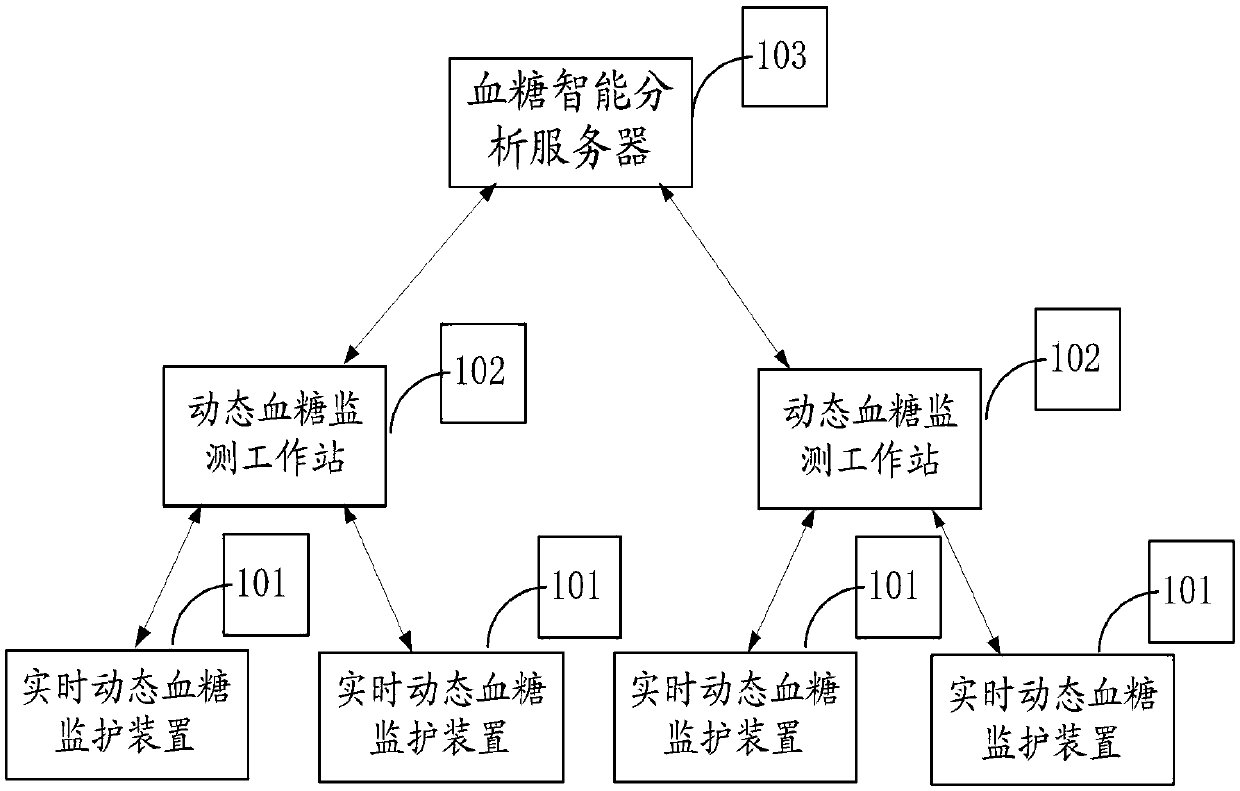
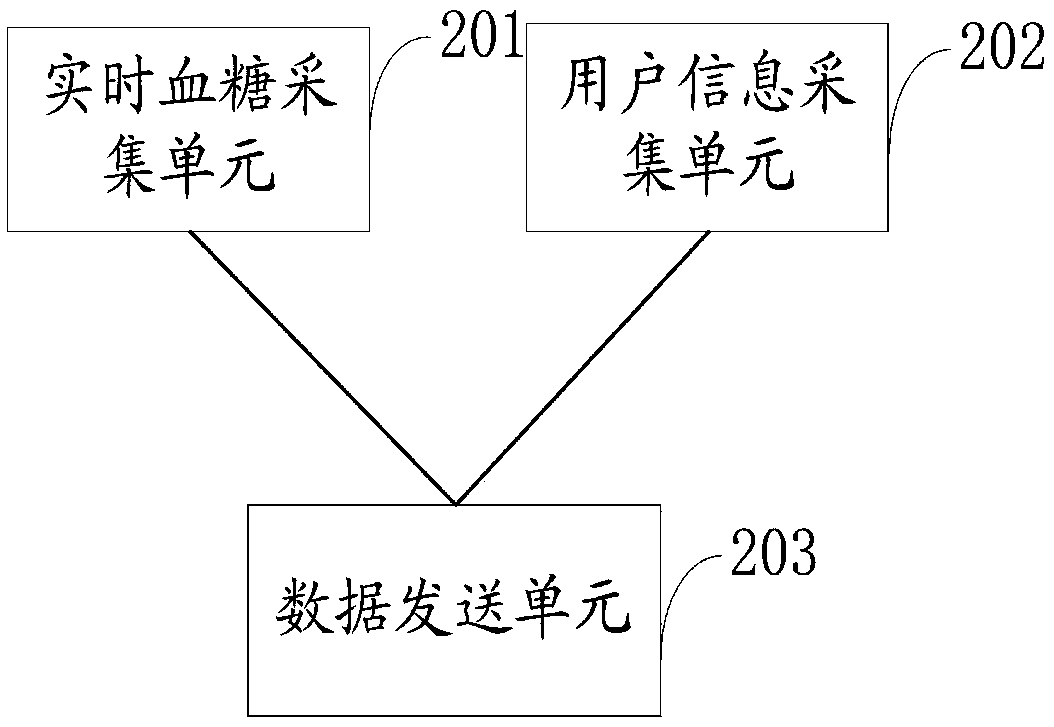
Type 2 diabetes monitoring system based on blood glucose monitoring and application in disease monitoring system
ActiveCN109637677AAnalytical intelligenceGood data supportMedical communicationSensorsDisease monitoringInsulin injection
This invention provides a type 2 diabetes monitoring system based on blood glucose monitoring, which comprises a real-time dynamic blood glucose monitoring device, a dynamic blood glucose monitoring workstation and a blood glucose intelligent analysis server. In combination with big data statistics, the type 2 diabetes monitoring system based on blood glucose monitoring presets a threshold group for judging blood glucose control situation, which corresponds to oral drug information or insulin injection information or insulin pump information, then a diagnostic analysis report of blood glucoseis generated by using the received user information and real-time blood glucose value according to preset rule, and the diagnostic analysis report of blood glucose is sent to the dynamic blood glucosemonitoring workstation to remind relevant personnel to make timely treatment and make intelligent and accurate analysis of blood glucose control in a long period, thereby providing good data supportfor subsequent blood glucose control. The type 2 diabetes monitoring system based on blood glucose monitoring has the advantages of being simple in structure and high in security. The invention further discloses an application of the type 2 diabetes monitoring system based on blood glucose monitoring in disease monitoring system.
Owner:北京中器华康科技发展有限公司
Rapid action insulin formulations and pharmaceutical delivery systems
ActiveUS9901622B2Fast absorptionStrict controlUltrasound therapyPeptide/protein ingredientsActive agentInfusion set
Owner:THERMALIN INC
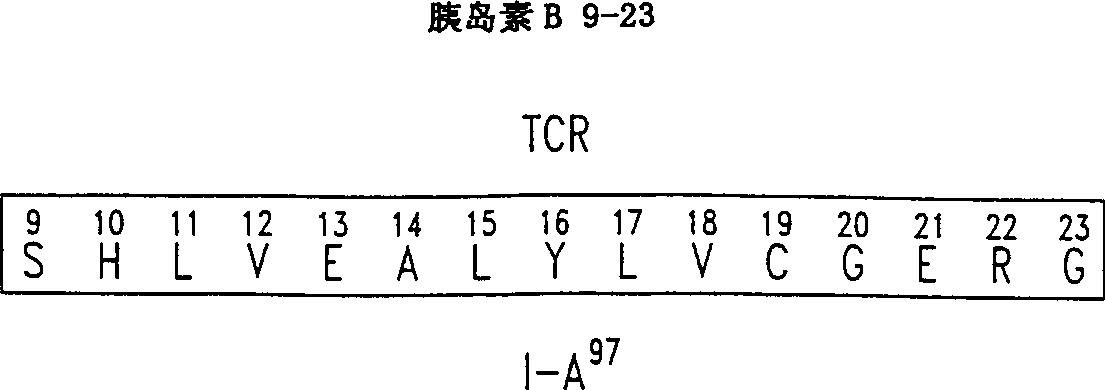
Methods for treatment of diabetes using peptide analogues of insulin
The present invention is directed toward peptide analogues of insulin (B) chain that are generally derived from peptides comprising residues (9 to 23) of the native (B) chain sequence. The analogues are altered from the native sequence at position (12, 13, 15) and / or (16), and may be additionally altered at position (19) and / or other positions. Pharmaceutical compositions containing these peptide analogues are provided. The peptide analogues are useful for treating and inhibiting the development of diabetes.
Owner:NEUROCRINE BIOSCI INC
Combination therapy for pi3k-associated disease or disorder
PendingCN113194752AEnhanced inhibitory effectOrganic chemistryPeptide/protein ingredientsDiseaseReceptor
Described herein are compositions and methods for treating a disease or disorder associated with PI3K signaling. For example, such compositions can include use of modulators of glucose metabolism, use of at least one kinase in the insulin-receptor / PI3K / AKT / mTOR pathway, and / or use of diet that influences the subject's metabolic state.
Owner:CORNELL UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com