Methods for treatment of diabetes using peptide analogues of insulin
An analog, diabetes technology, applied in the field of diabetes treatment, can solve problems such as short half-life of insulin and difficult dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of peptides
[0051] This example illustrates the synthesis of representative peptide analogs.
[0052] The peptides were synthesized on a peptide synthesizer (Beckman Model 990) by solid phase method. A p-methylbenzylamine resin (MBHA resin) was used to prepare peptides with amidated carboxyl ends; for peptides with free carboxyl ends, a Merrifield resin coupled with appropriately protected amino acids was used. These two resins were purchased from Bachem Fine Chemicals (Torrance, CA). Unless otherwise specified, the derivatized amino acids (Bachem Fine Chemicals) used in the synthesis are all in the L-configuration, and the N-α-amino function is protected with tert-butoxycarbonyl without exception. The side chain functional groups are protected as follows: benzyl protects serine and threonine; cyclohexyl protects glutamic acid and aspartic acid; p-toluenesulfonyl protects histidine and arginine; 2-chlorobenzyloxycarbonyl protects lys Amino...
Embodiment 2
[0054] Long-term T cell line
[0055] This example illustrates the preparation of an insulin-specific long-term NOD T cell line.
[0056] When there are diffuse NOD splenocytes as antigen-presenting cells and cytokines, they are stimulated in vitro with 25μg / ml porcine insulin or diffused NOD islet cells, and the lymphocytes isolated from the islet infiltration population are cultured to establish insulin-specific NOD T cell line. In order to obtain the infiltrated lymphocytes, the following operations (see Wegmann et al., European Journal of Immunology 24: 1853, 1994): digest the pancreas of NOD mice with collagenase and manually separate individual islets. Then the islets are gently digested by trypsin to obtain infiltrated lymphocytes. When NOD spleen cells, porcine insulin and lymphokines are present, a series of stimulations are performed to proliferate insulin-specific T cell lines or clones. These clones were obtained by limiting dilution of a T cell line specifi...
Embodiment 3
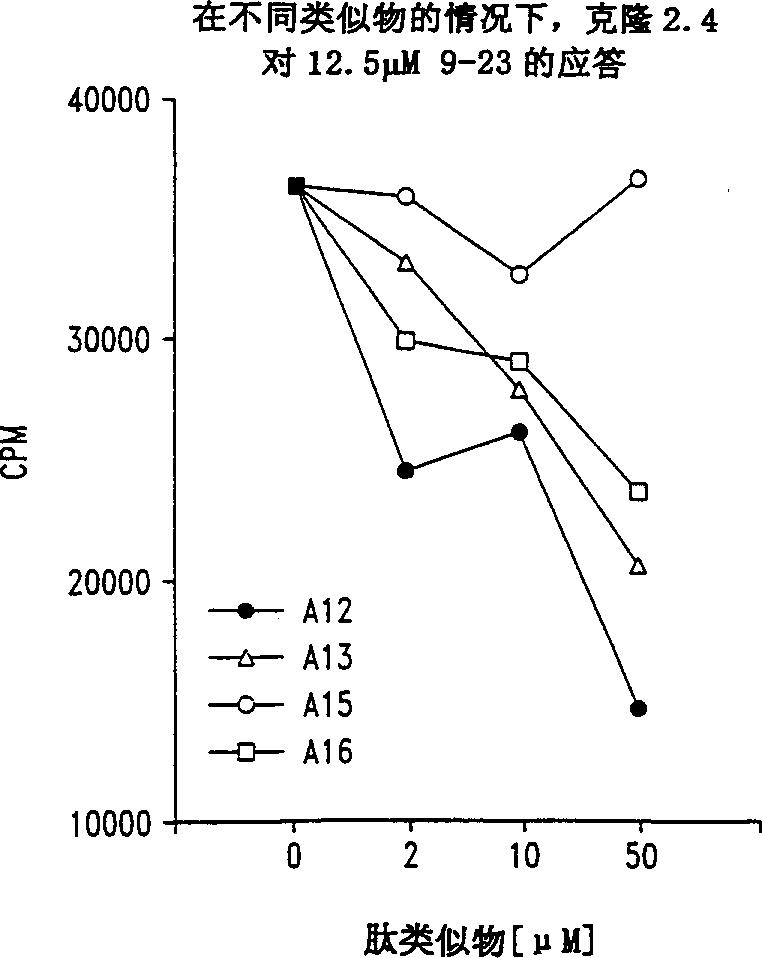
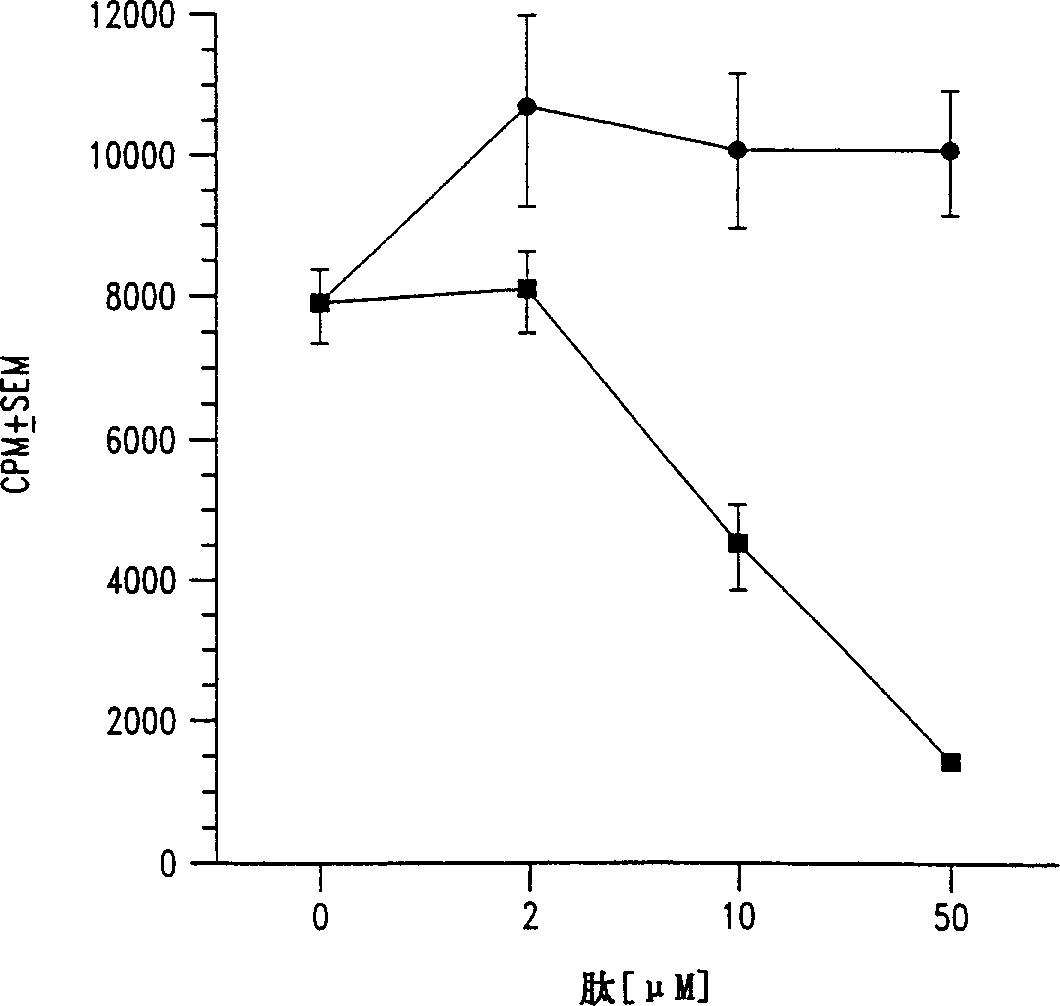
[0058] Effect of peptide analogs on the clonal proliferation of insulin-specific NOD T cells
[0059] This example illustrates the effect of representative peptide analogs on T cell proliferation.
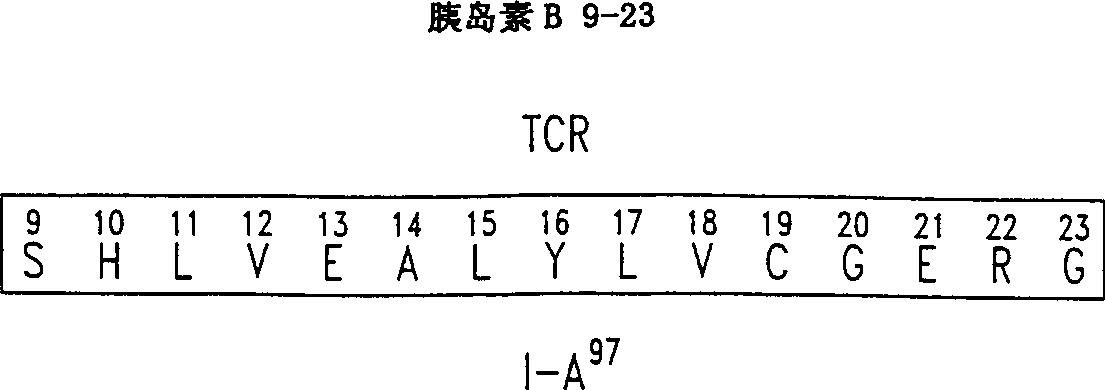
[0060] As described in Example 2, a mouse (NOD) T cell clone specific for insulin B chain (9-23) (SEQ ID NO: 2) was isolated from the infiltrated islets. The peptide analogs containing single alanine substitution were prepared as described in Example 1. The effects of each analogue on T cell proliferation were then evaluated using tests performed in 96-well flat-bottomed microtiter plates (see Daniel et al., European Journal of Immunology, 25: 1056, 1995). Simply put, in the presence of 50μg / ml insulin B chain 9-23 peptide or any of the alanine substituted peptides listed below, culture three 25,000 T cell clones and one million diffuse NOD splenocytes . At 7% CO 2 Incubate the titer plate in an atmosphere for a total of 72 hours, and pulse with 1μCi / well of tritiated thymidine for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com