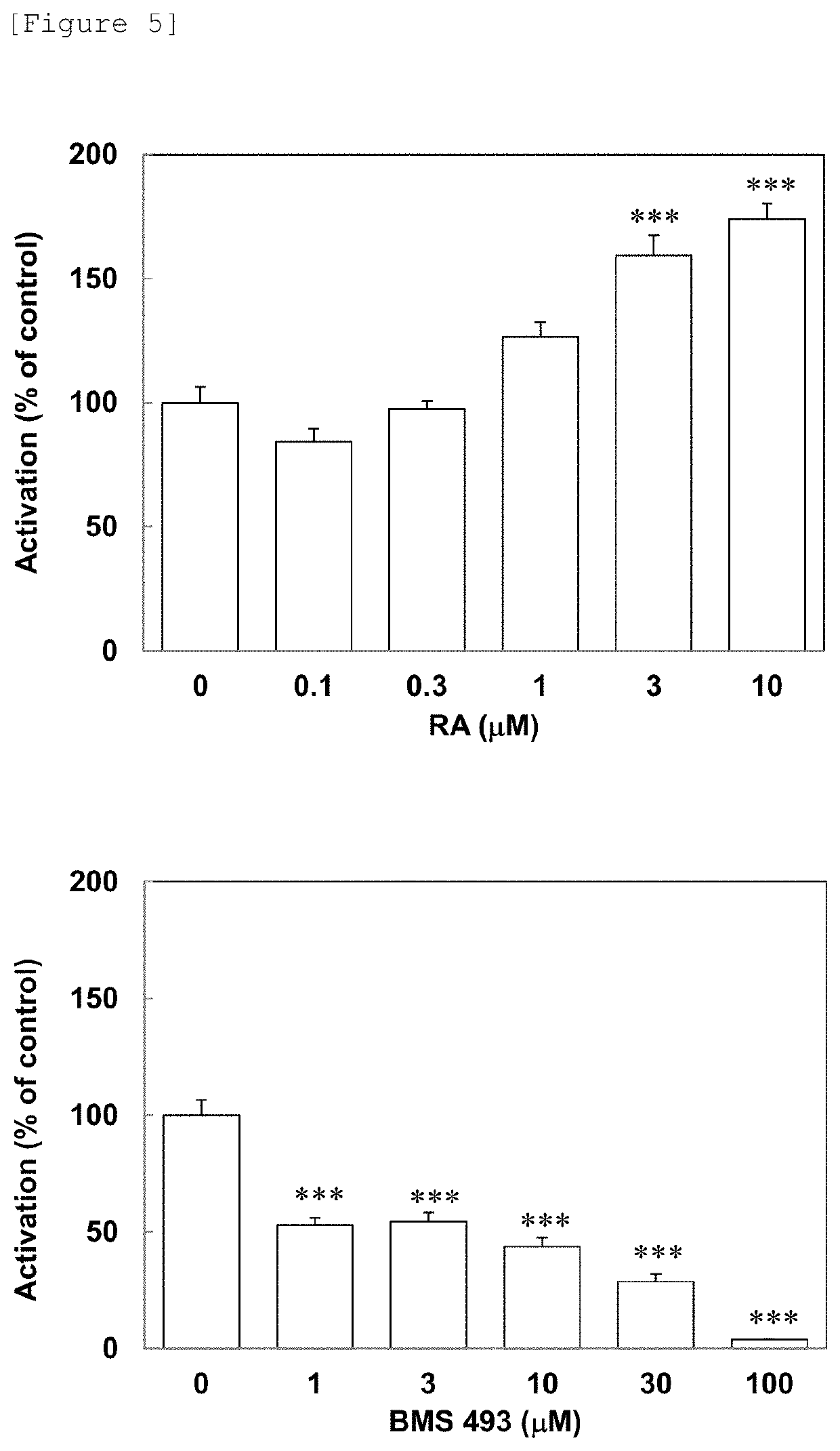
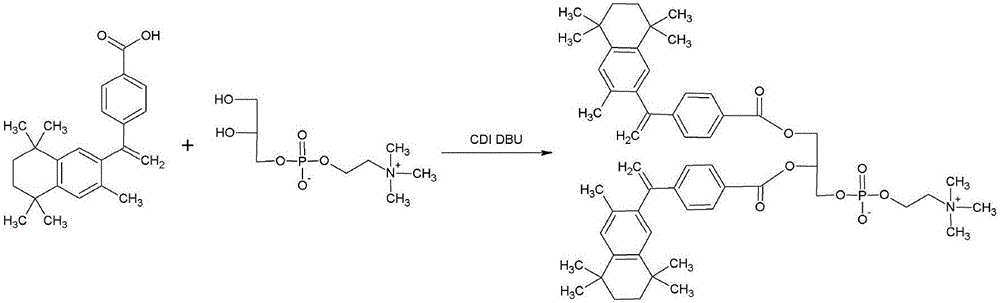
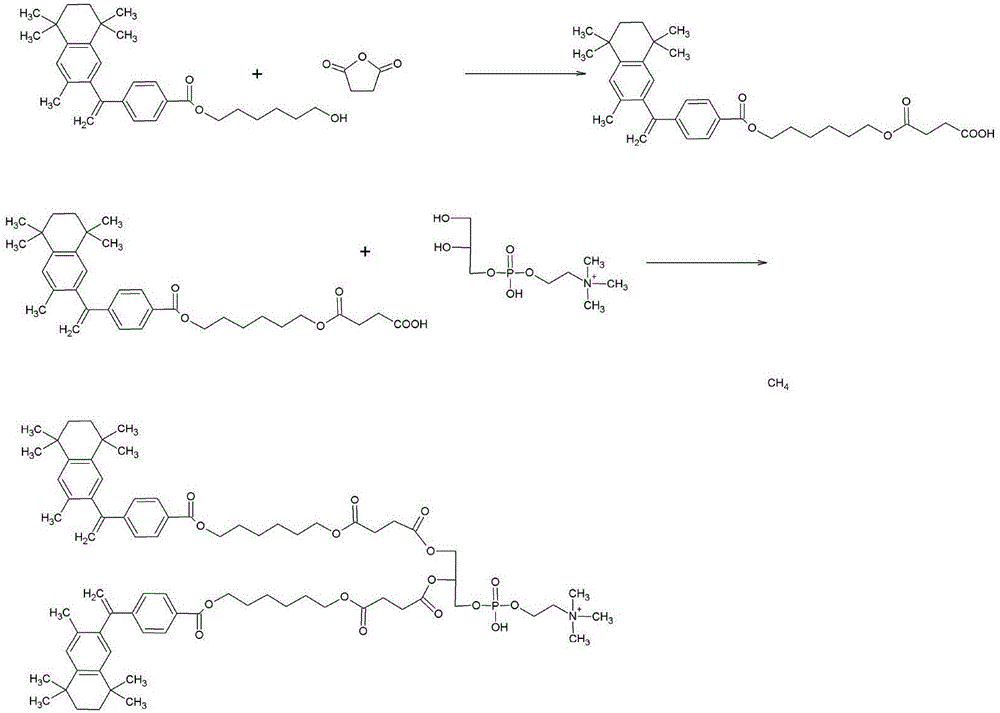
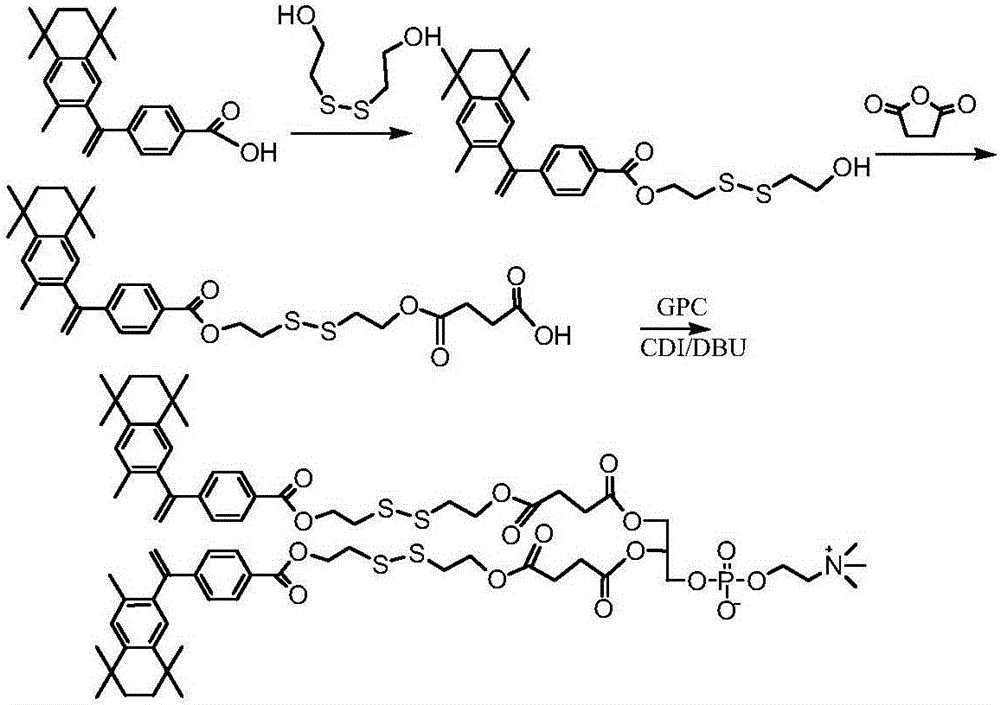
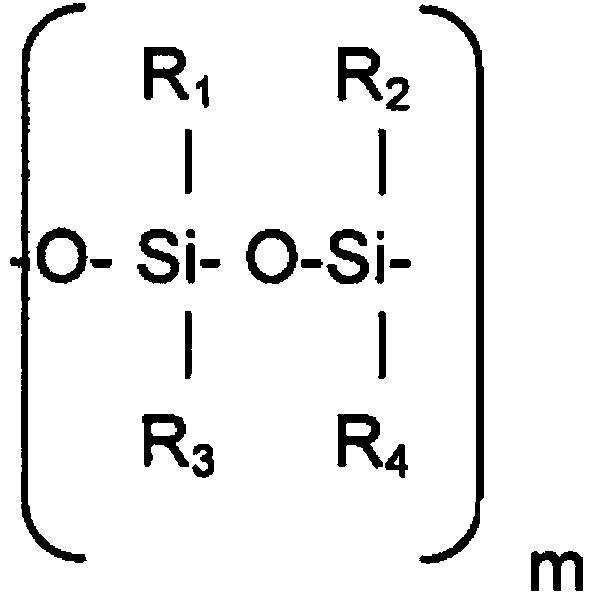
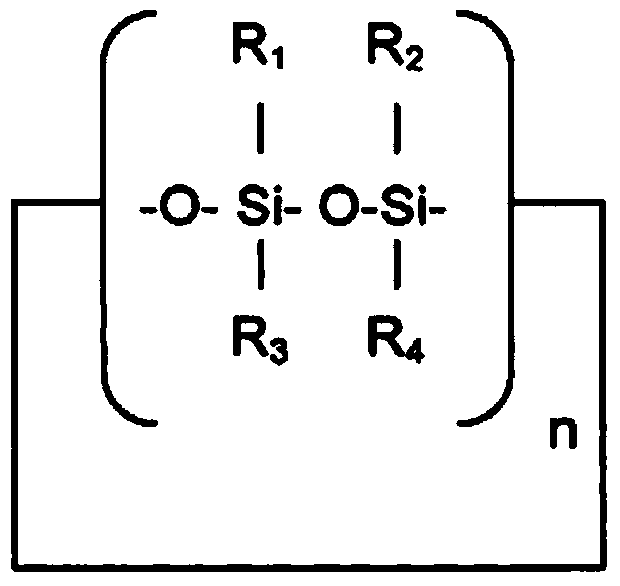
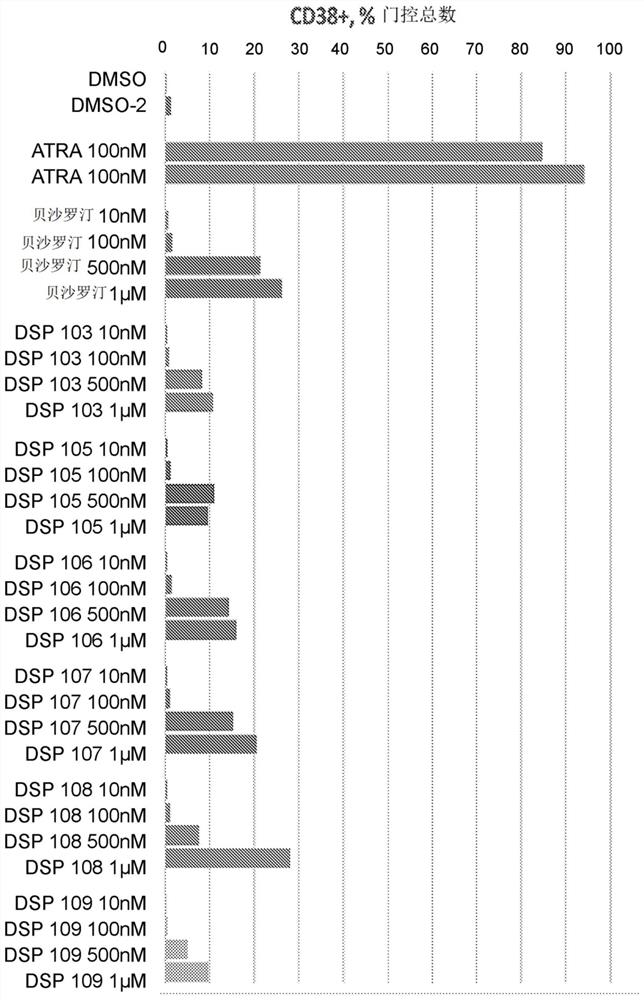
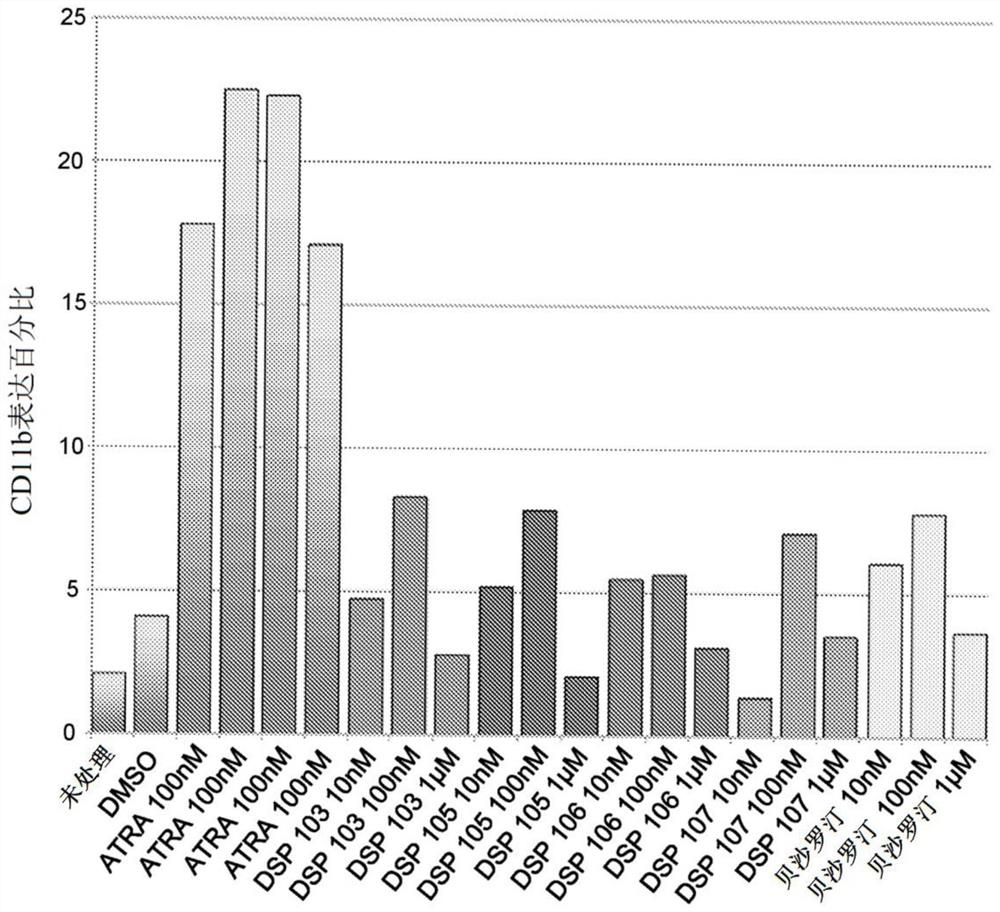
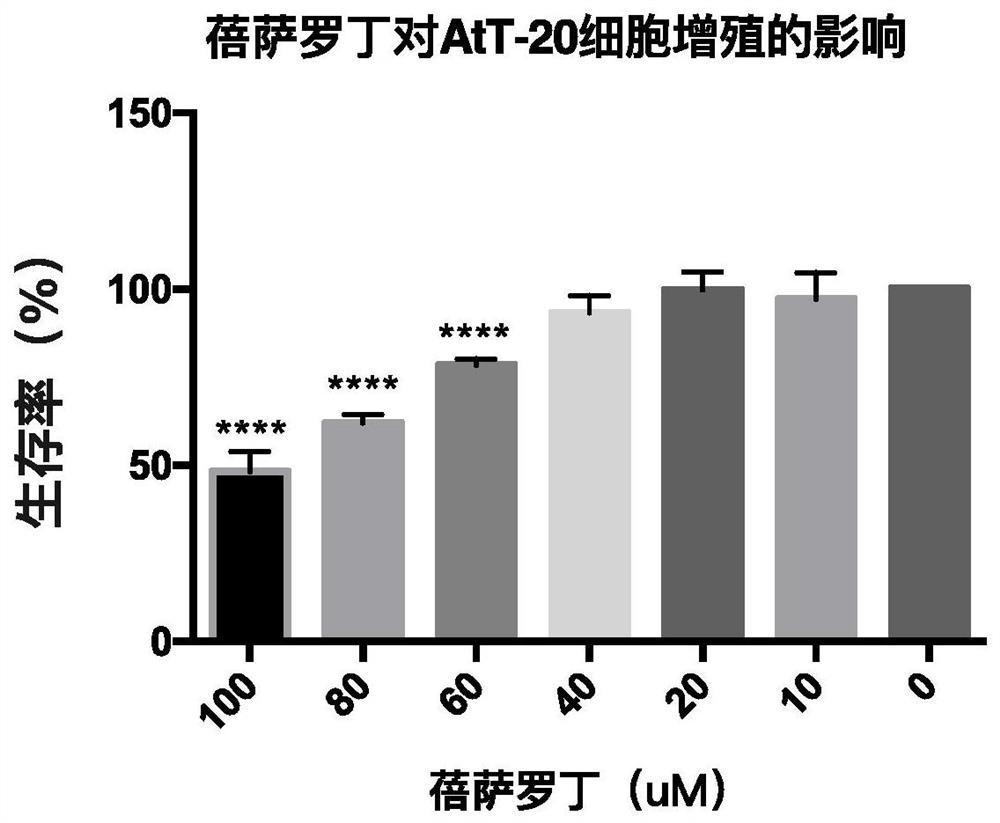
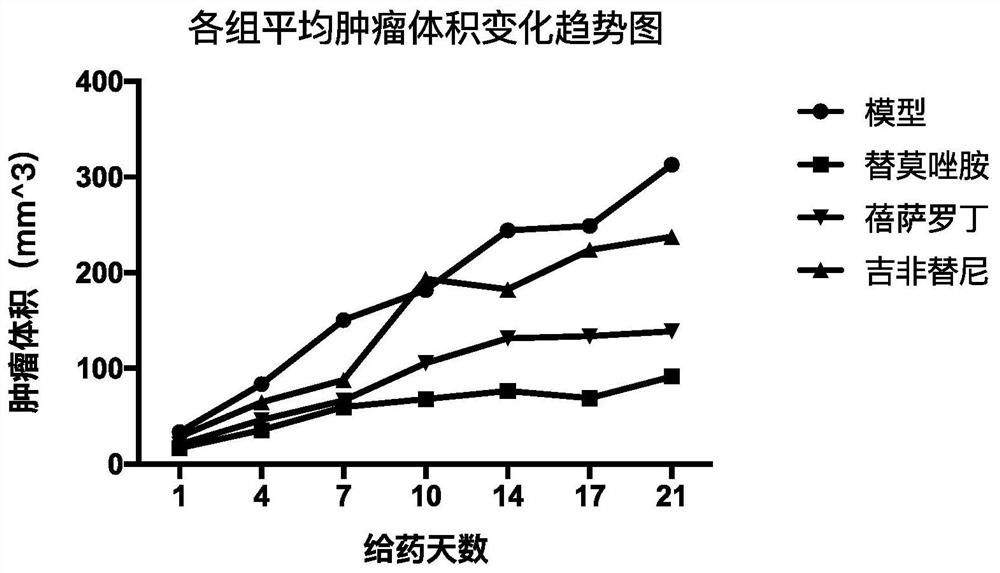
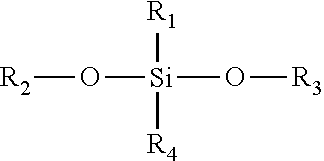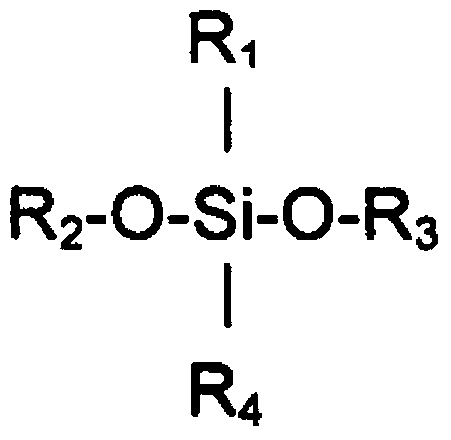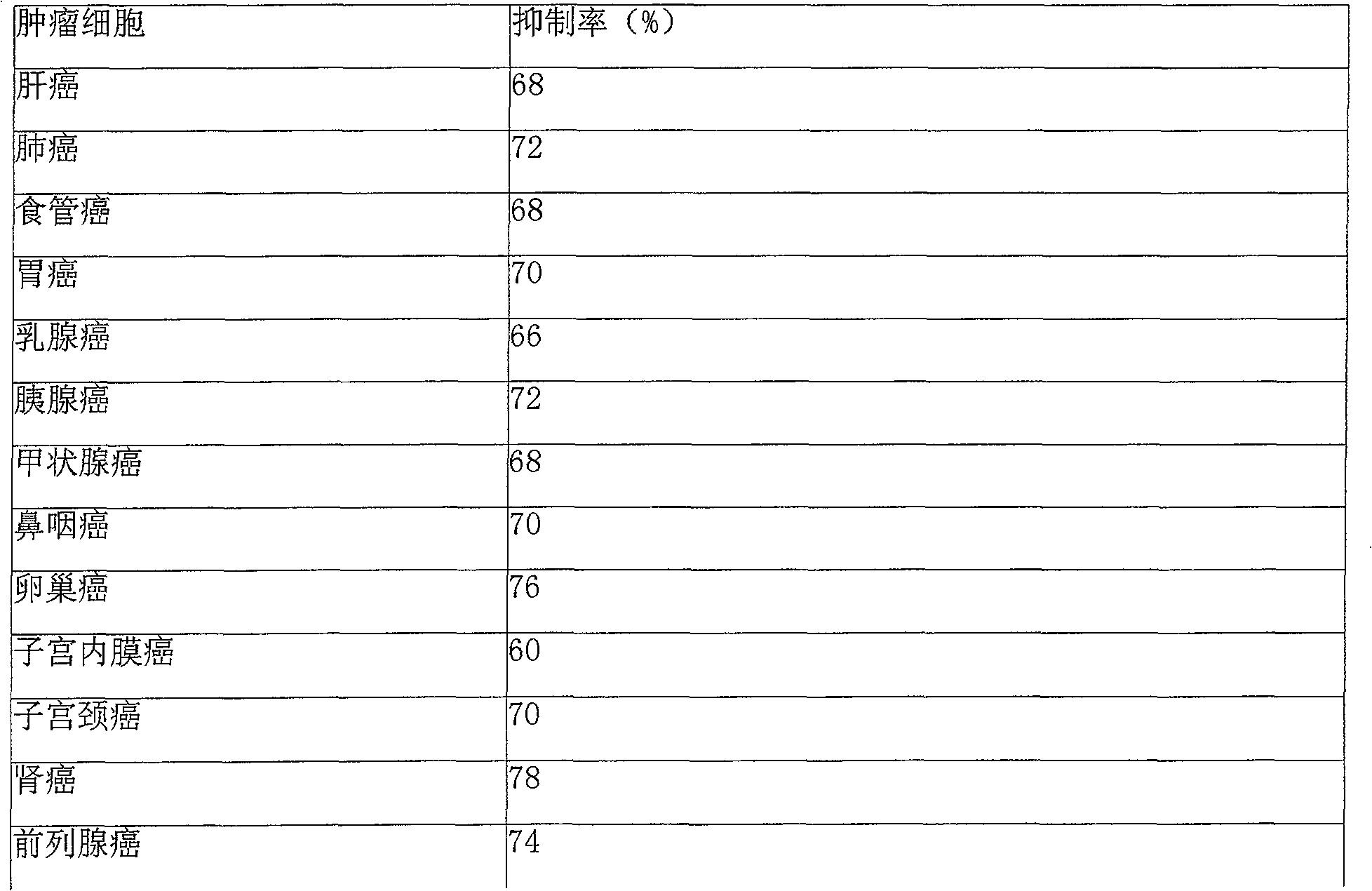Patents
Literature
32 results about "Bexarotene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
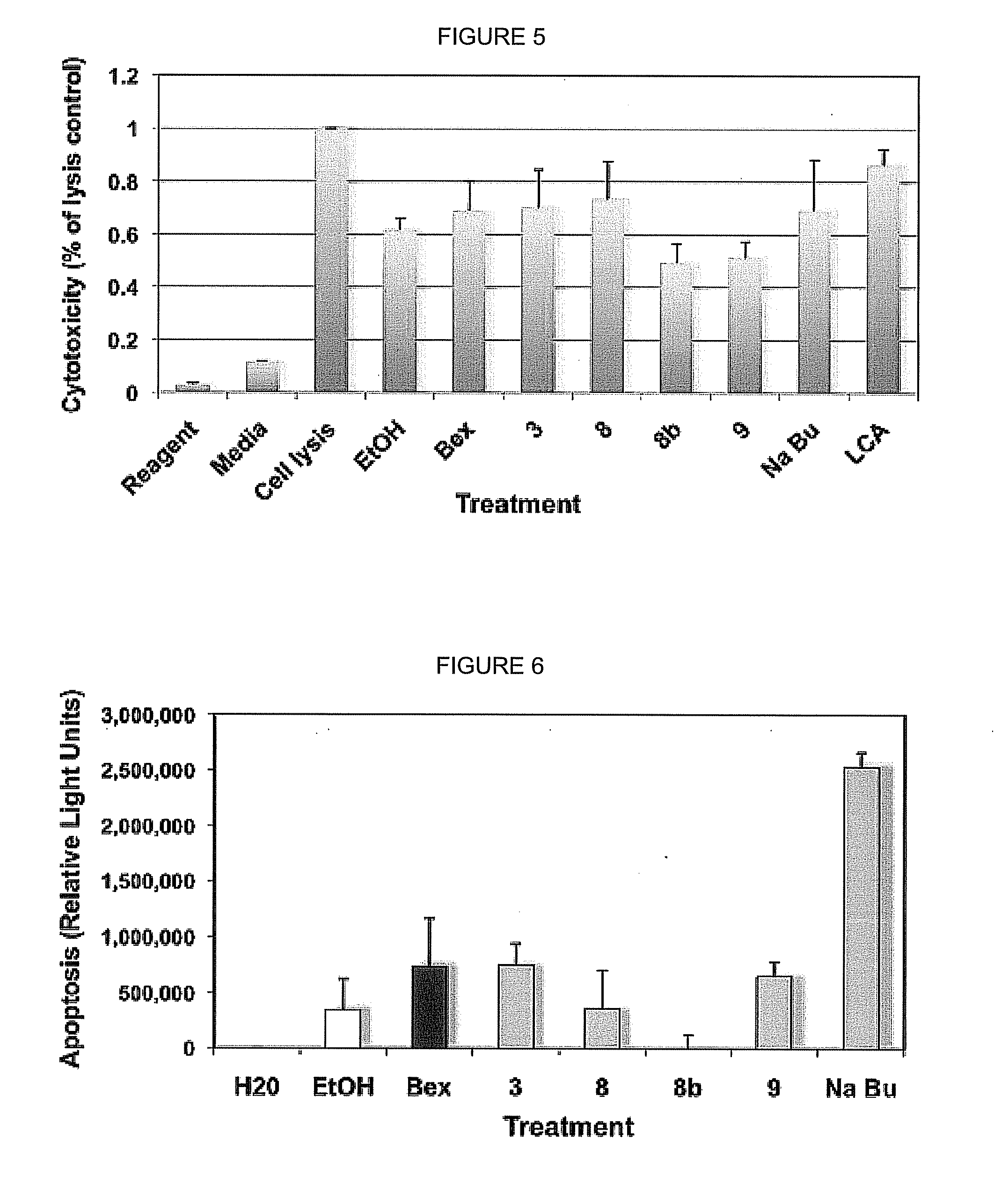
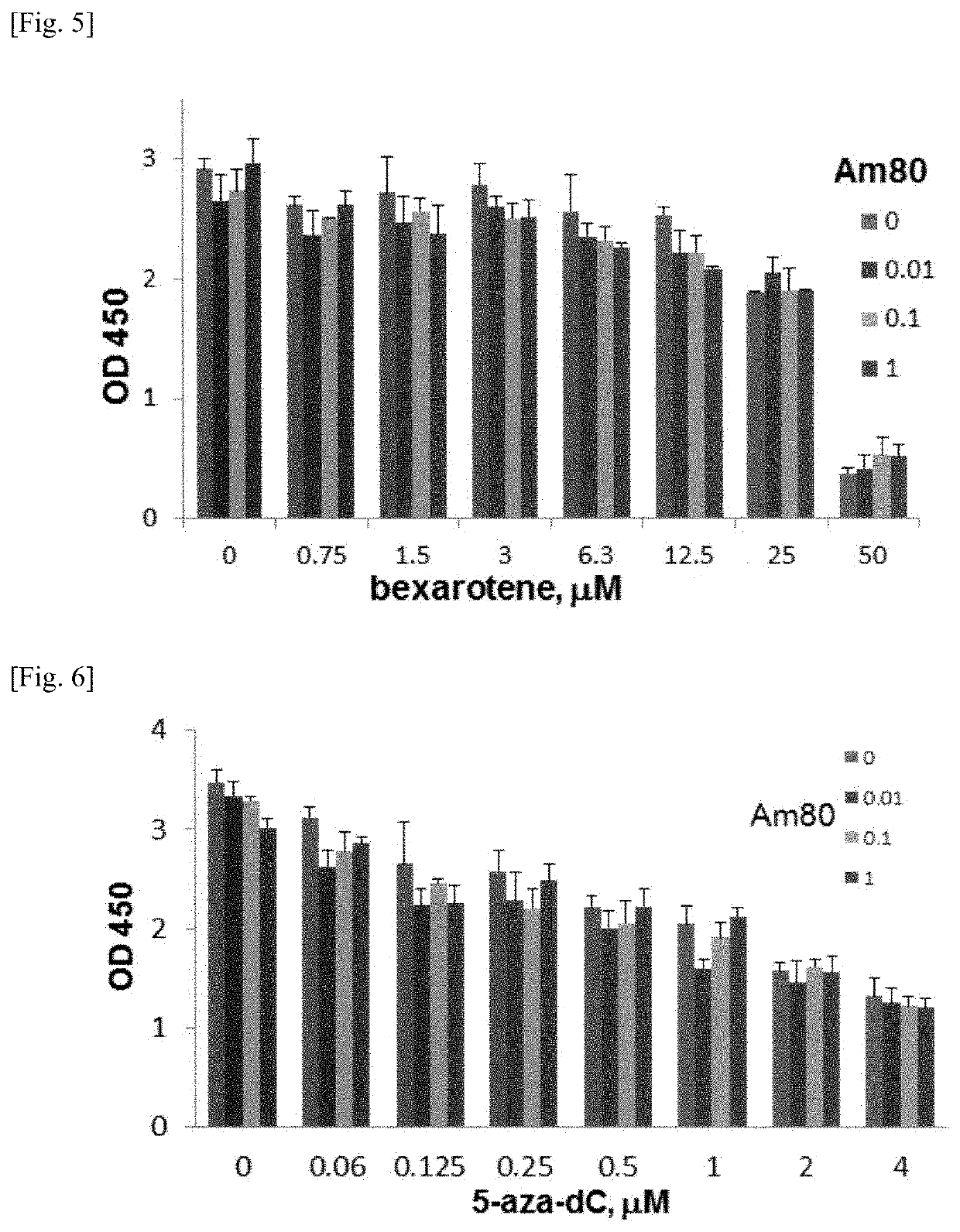
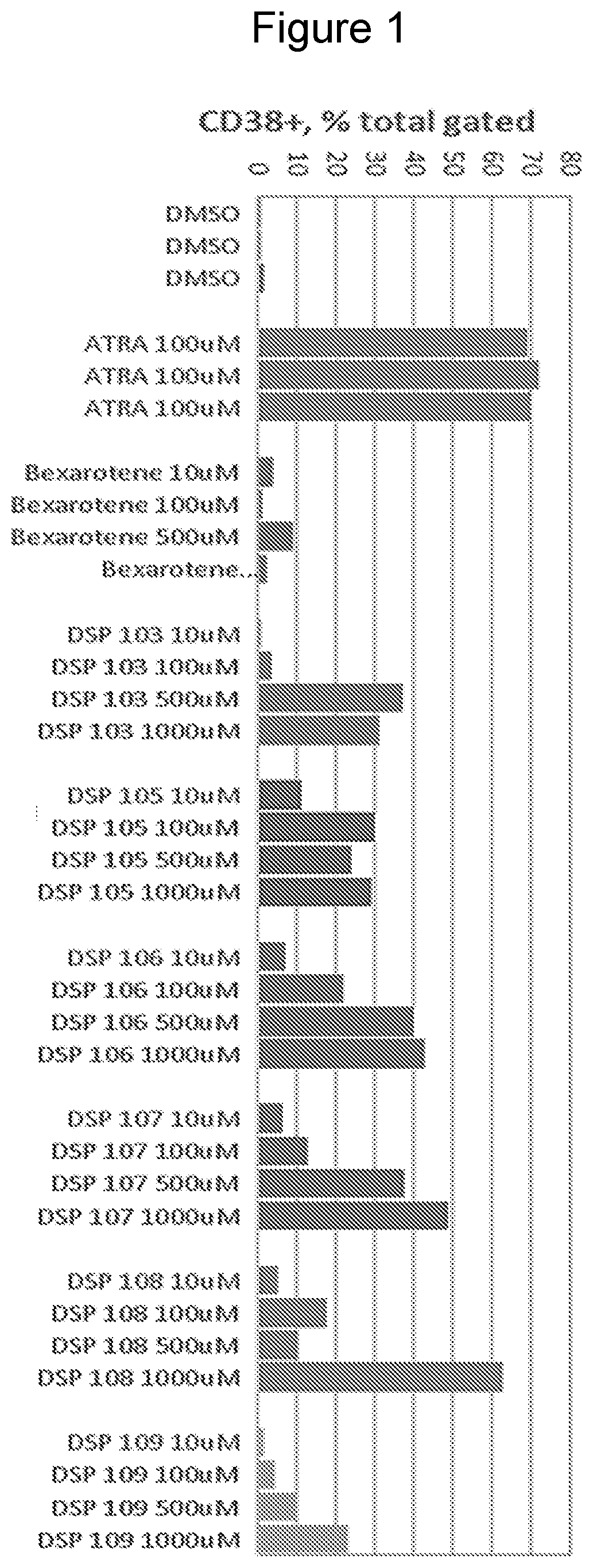
Bexarotene is used to treat skin problems from a certain type of cancer (cutaneous T-cell lymphoma-CTCL).
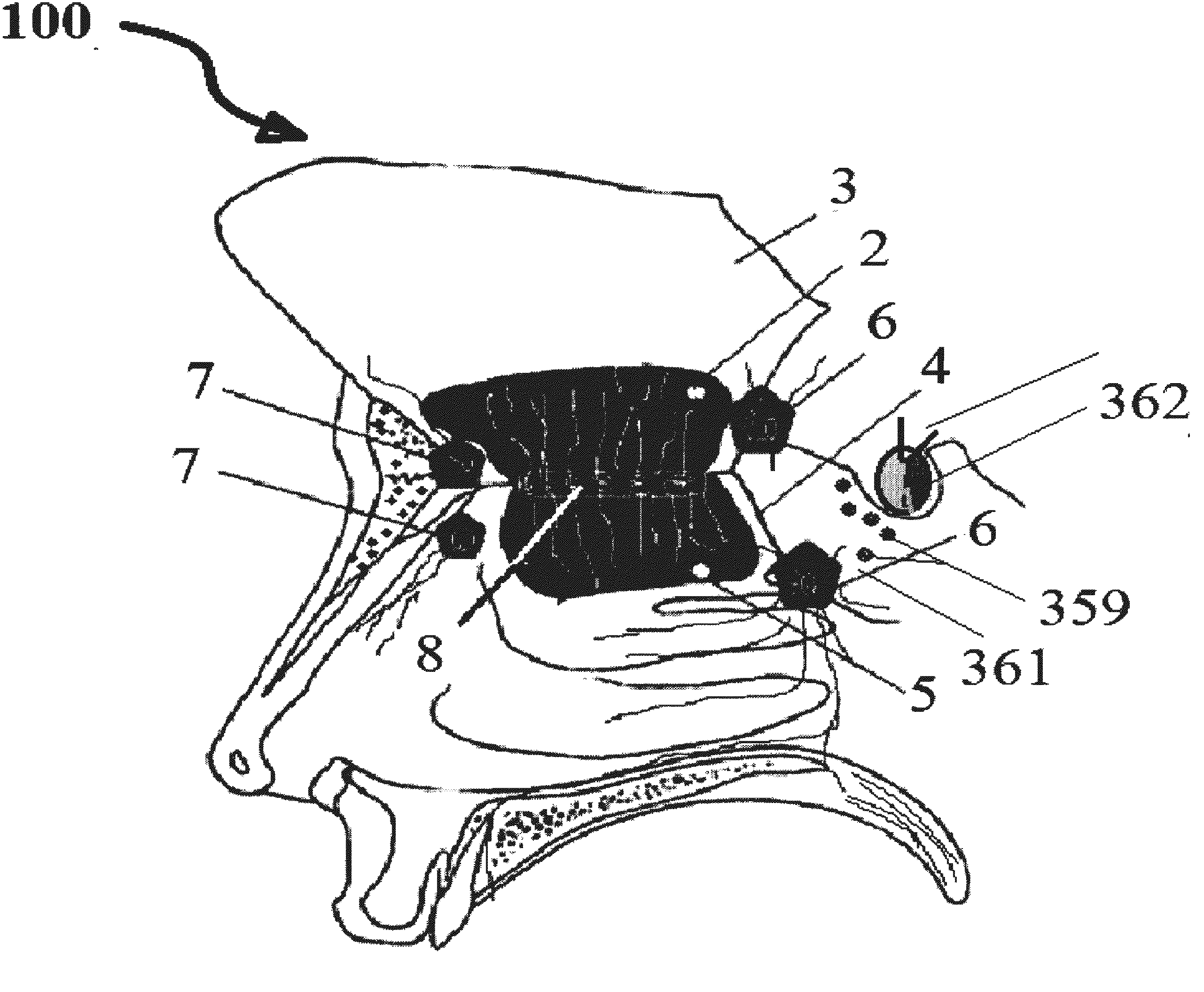
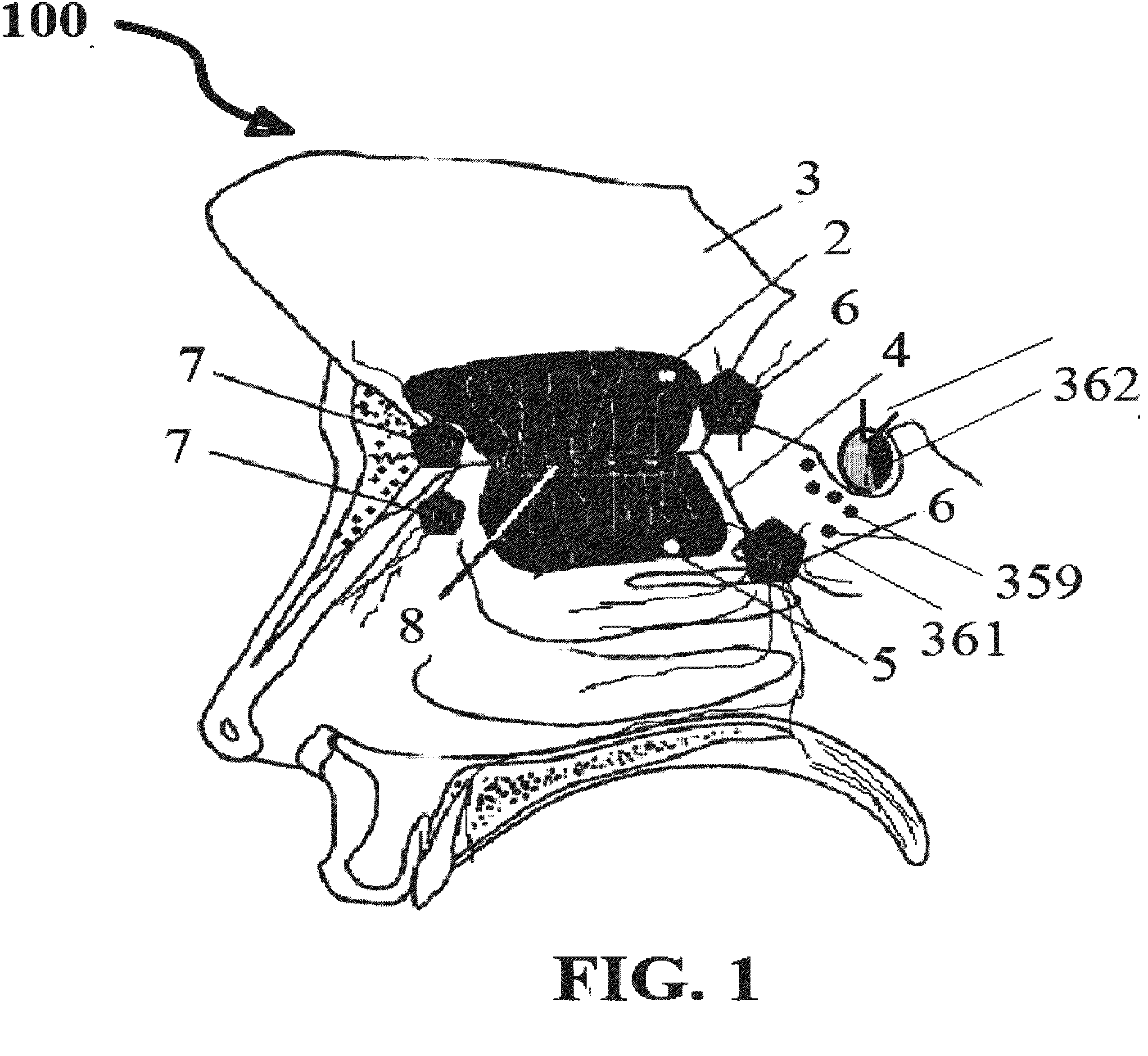
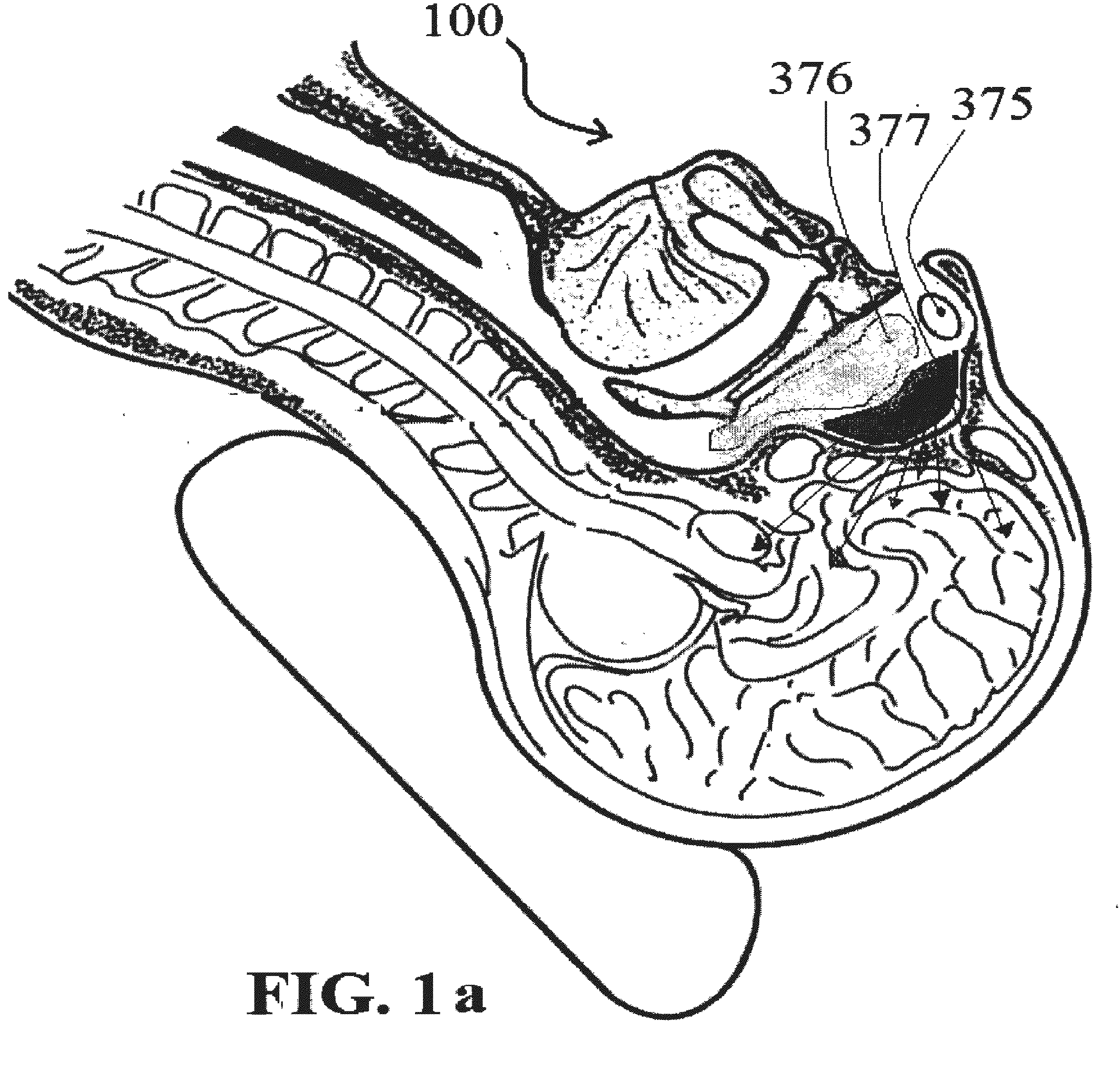
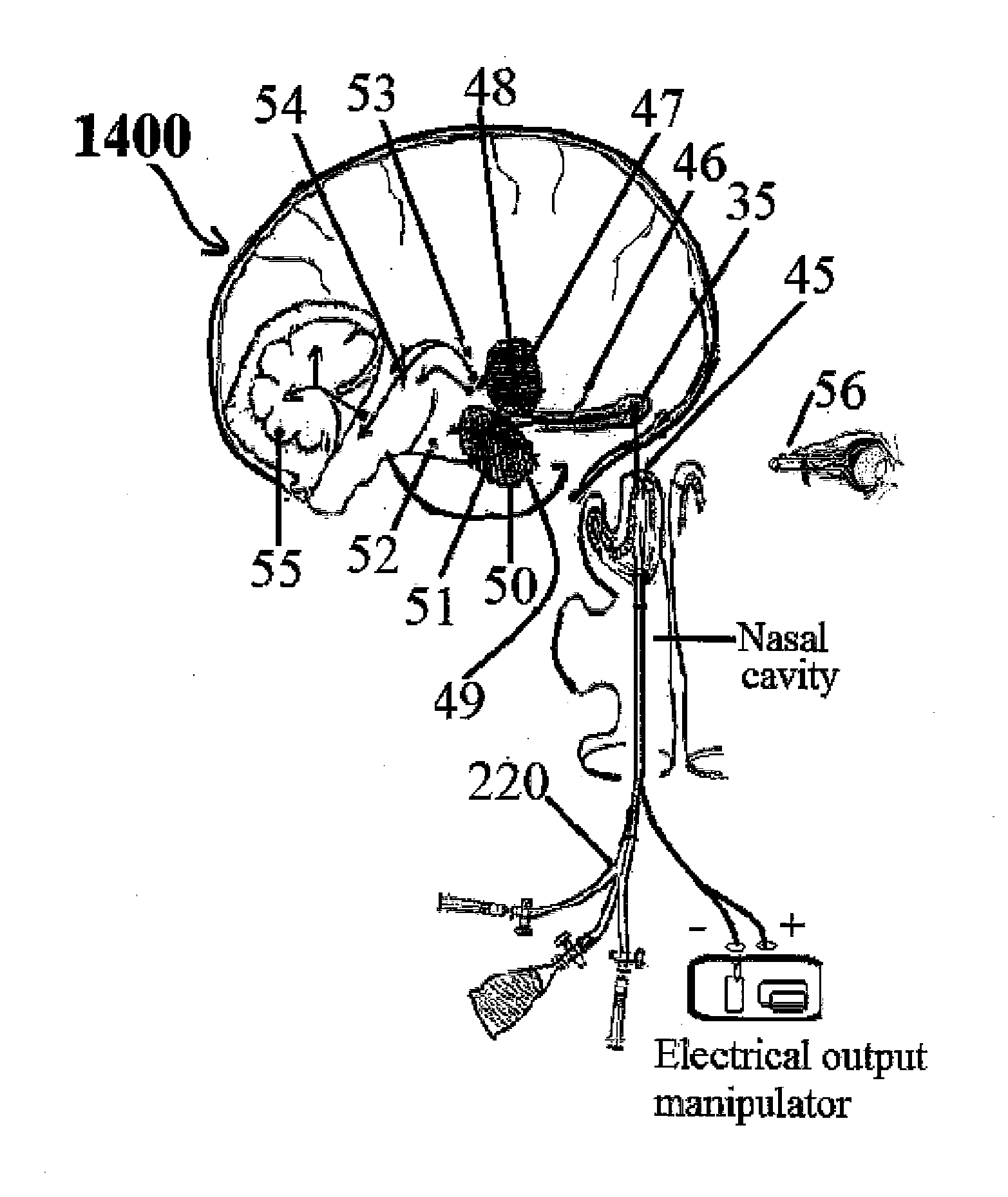
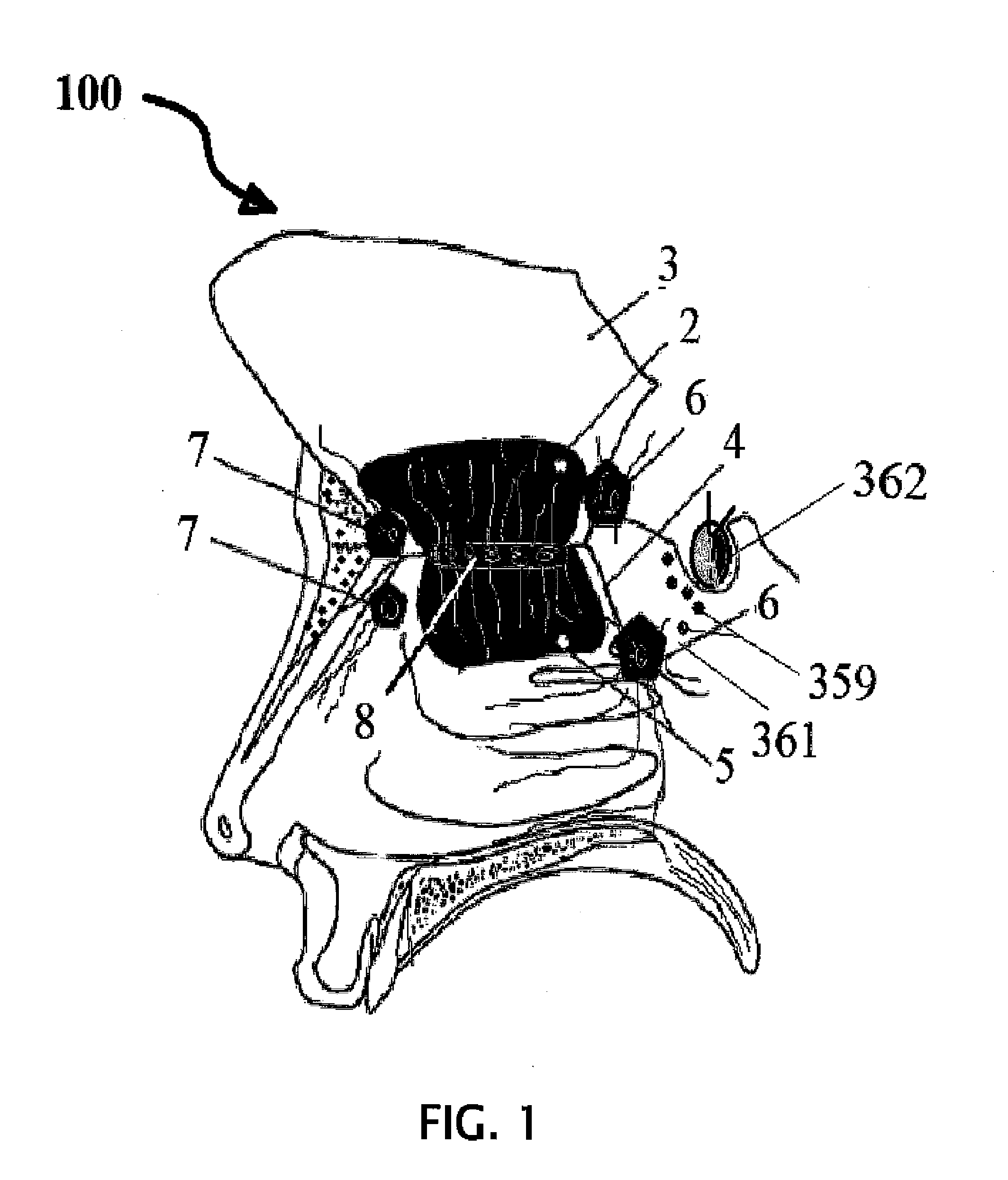
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20140012182A1Large deliveryAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Novel bexarotene analogs
Owner:ARIZONA STATE UNIVERSITY
Topical pharmaceutical compositions comprising bexarotene and a corticosteroid
Topical pharmaceutical compositions are described comprising: (a) bexarotene; (b) a corticosteroid; and (c) a carrier or vehicle. Said compositions are useful for the treatment of skin disorders.
Owner:ALMIRALL
Methods and compositions of predicting activity of retinoid x receptor modulator
ActiveUS20150368720A1Chemical property predictionCompound screeningGenomic BiomarkerGenetic linkage disequilibrium
The present invention describes genomic biomarkers that have been discovered to correlate with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic retinoid X receptor modulator, such as bexarotene, in treating diseases such as, non small cell lung cancer. The newly discovered biomarkers and others in linkage disequilibrium with them can be used in companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
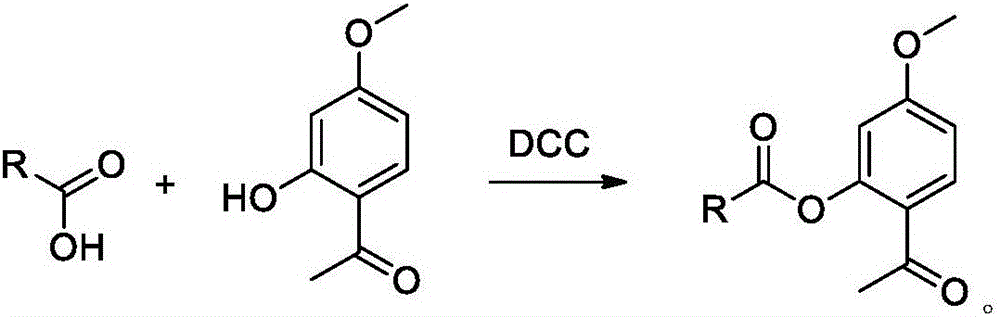
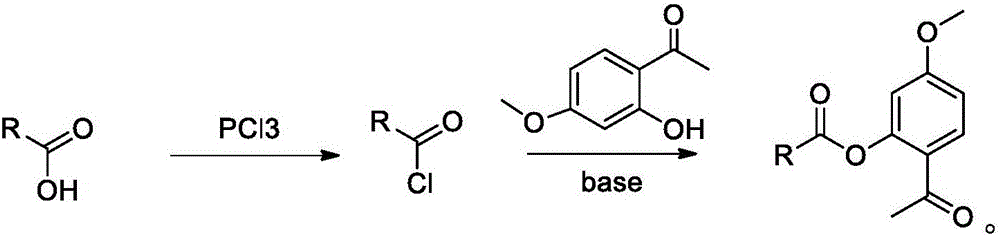
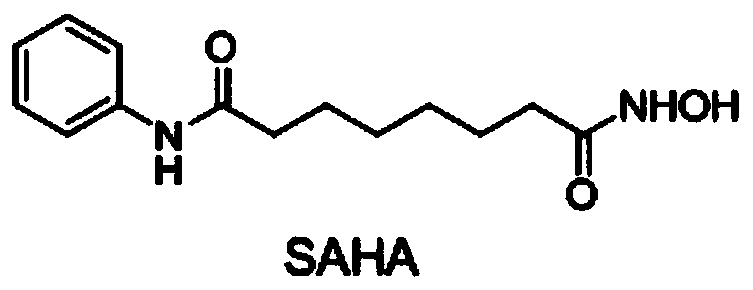
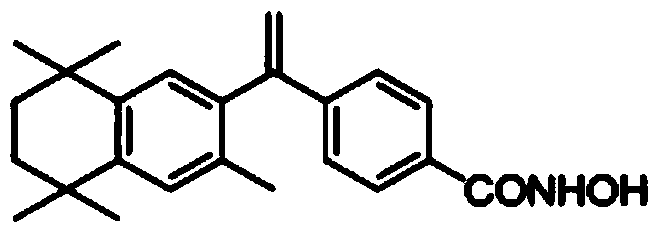
Bexarotene hydroximic acid as well as preparation method and application thereof
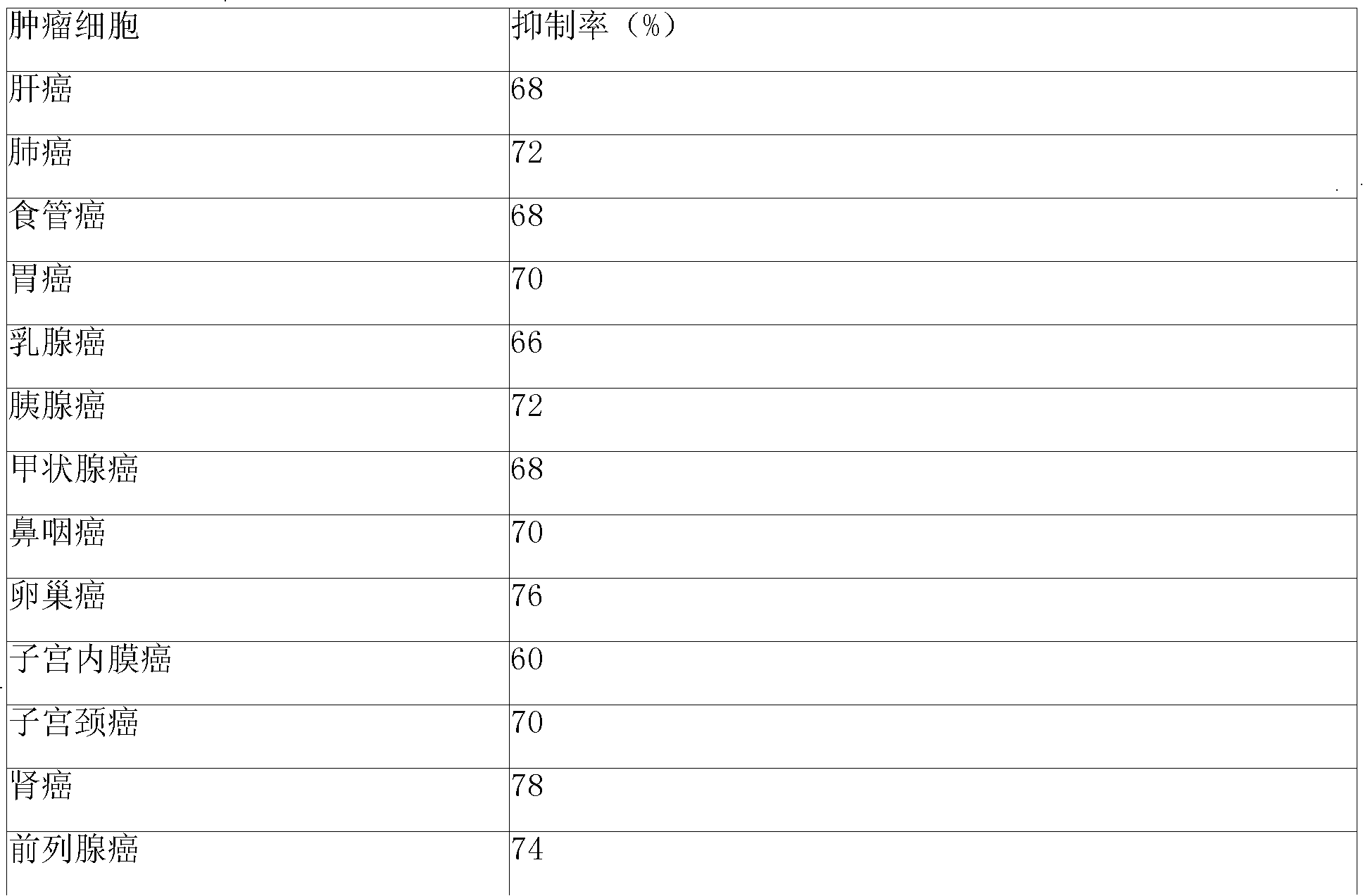
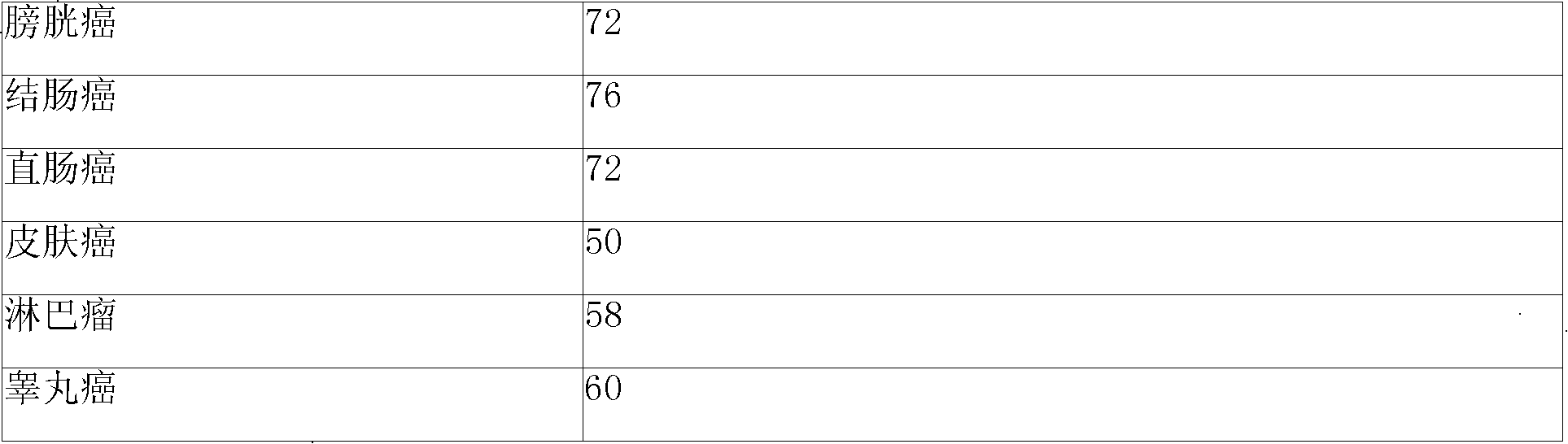
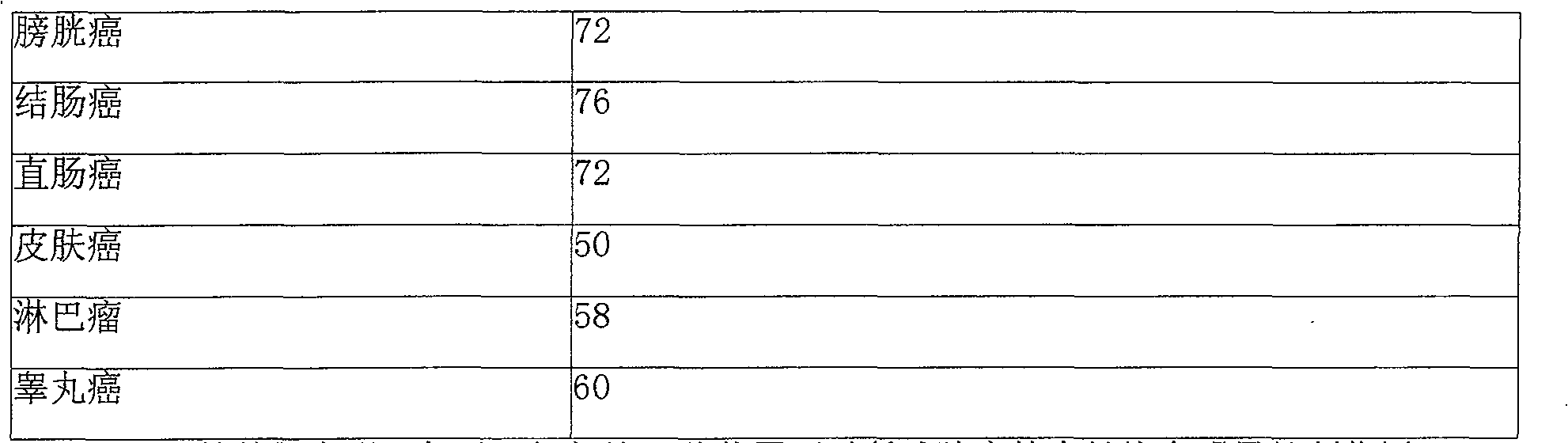
InactiveCN102503857AHas anti-tumor effectEasy to prepareOrganic chemistryAntineoplastic agentsHydroxizinumCancer cell
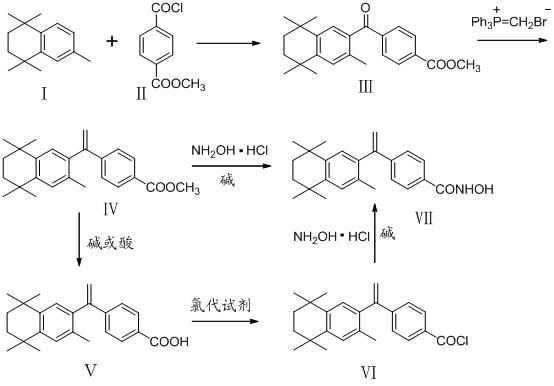
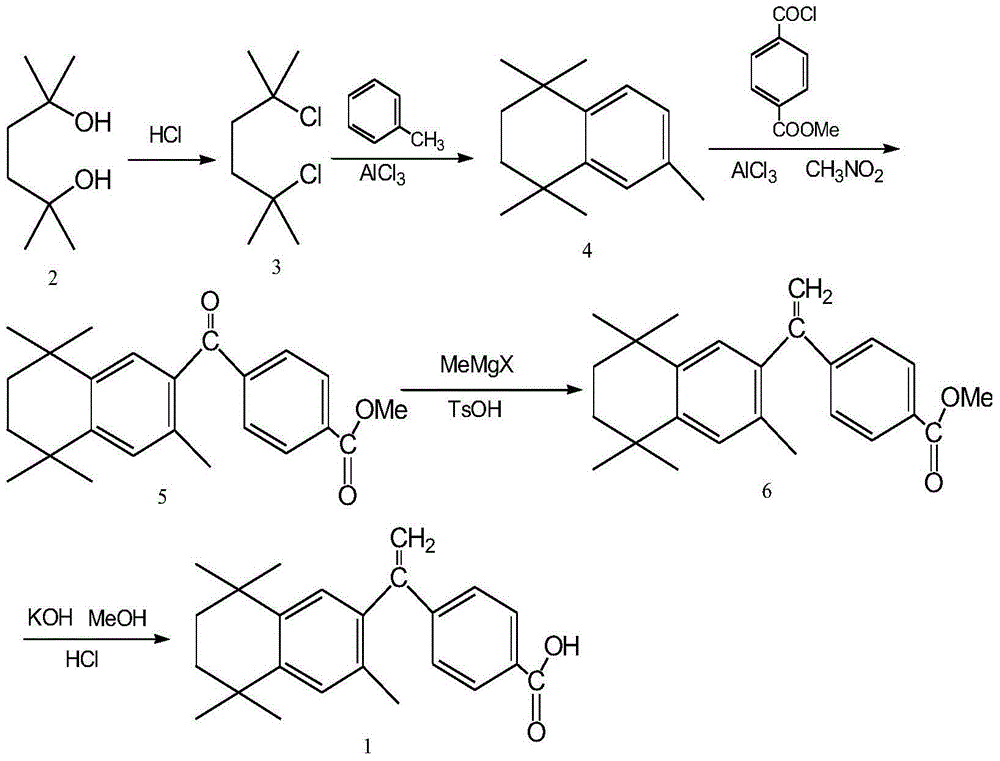
The invention belongs to the technical field of medicine and discloses bexarotene hydroximic acid as well as a preparation method and application thereof. The formation of the compound is as shown in the picture and the objective compound is prepared mainly by taking 1,1,4,4,6- pentamethyl-1,2,3,4-tetrahydronaphthalene as the initial material through the steps of Friedel-Crafts acylation, Wittig reaction and condensation reaction. The method is simple in operation, convenient in post-processing and high in yield. The objective compound has good inhibitory action on various cancer cells by the multiple action mechanisms of bonding the retinoic acid X receptors and restraining the histone deacetyltransferases, and the anti-cancer activity of the bexarotene hydroximic acid is obviously better than that of bexarotene. Therefore, the bexarotene hydroximic acid can be applied to treatment of cancers.
Owner:SHENYANG PHARMA UNIVERSITY
Bexarotene analogs
Owner:ARIZONA STATE UNIVERSITY
Vitamin A compound and paeonol condensed derivatives and preparation method
InactiveCN106631950ALess irritatingPromote differentiationOrganic compound preparationCarboxylic acid esters preparationIrritationCutin
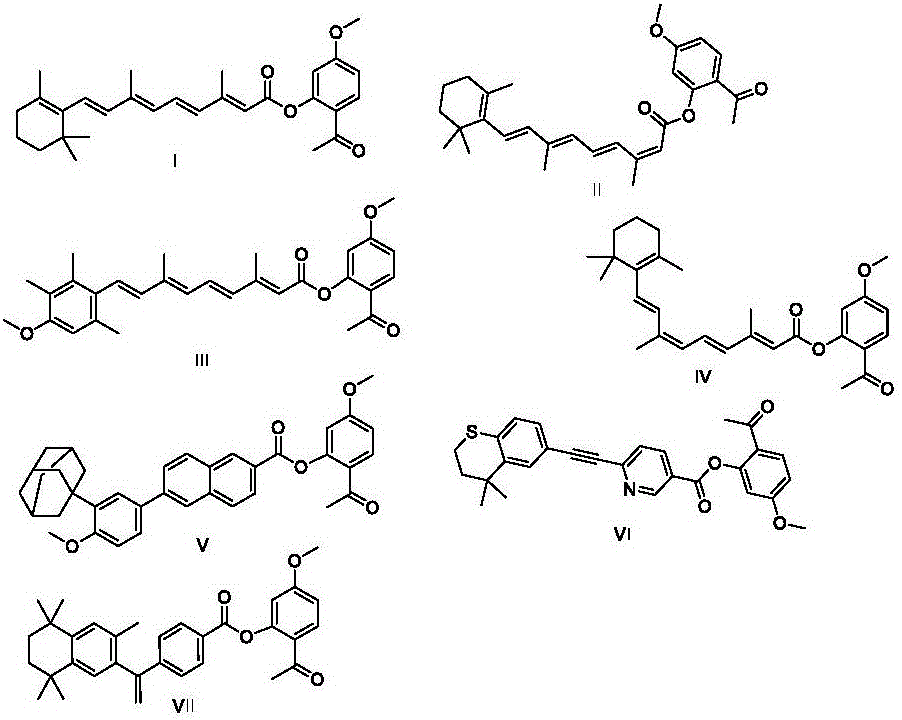
The invention discloses vitamin A compound and paeonol condensed derivatives and a preparation method. The vitamin A compound and paeonol condensed derivatives include derivatives in structures I-VII shown as follows; a structure I is ester formed by paeonol and all-trans retinoic acid; a structure II is ester formed by paeonol and 13-cis-retinoic acid; a structure III is ester formed by paeonol and acitretin; a structure IV is ester formed by paeonol and 9-cis-retinoic acid; a structure V is ester formed by paeonol and adapalene; a structure VI is ester formed by paeonol and tazarotene; a structure VII is ester formed by paeonol and bexarotene. By the vitamin A compound and paeonol condensed derivatives and the preparation method, irritation of vitamin A compounds to skin mucosa is relieved; after application to skin, the vitamin A compounds and paeonol are released under the action of hydrolase to dissolve cutin, promote epithelial cell differentiation and the like so as to realize anti-inflammatory and anti-oxidation functions.
Owner:苏州药基美研医药科技有限公司
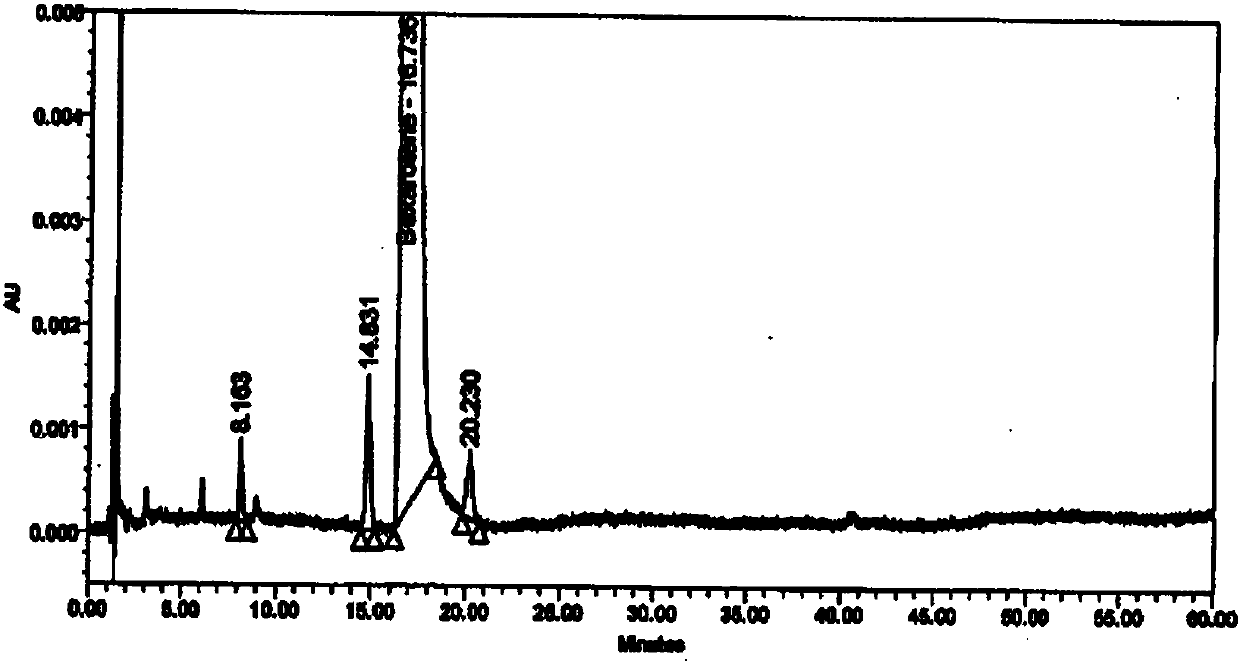
Method for detecting impurity in bexarotene softgel by high performance liquid chromatography
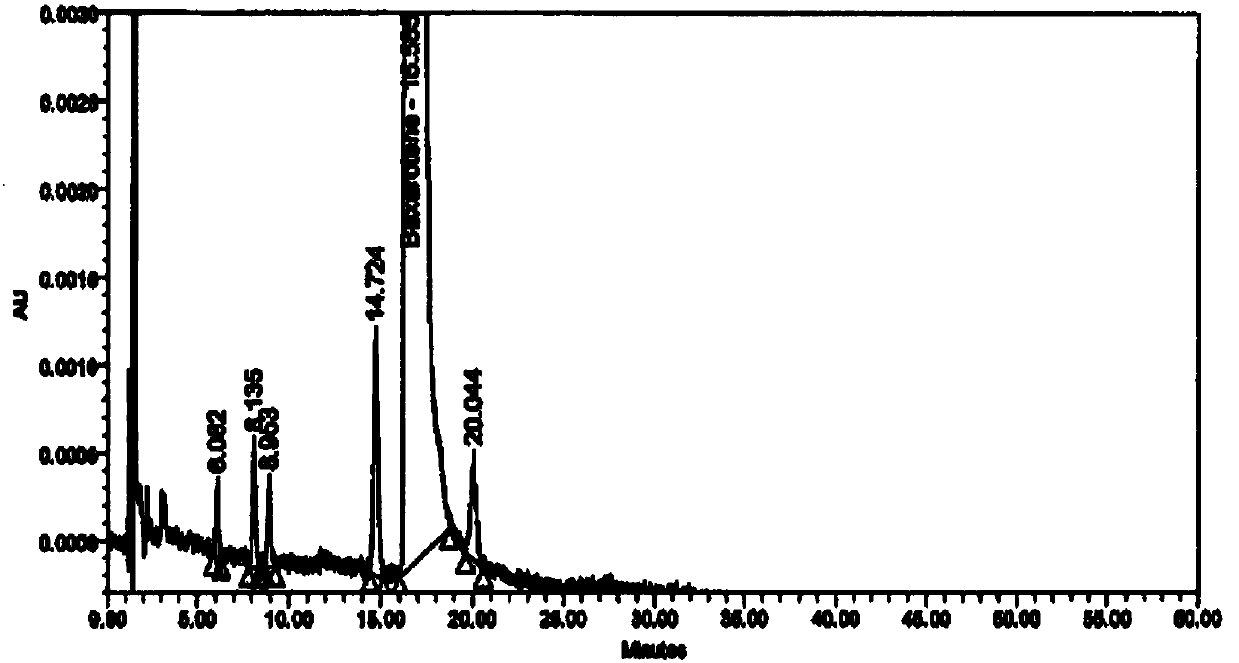
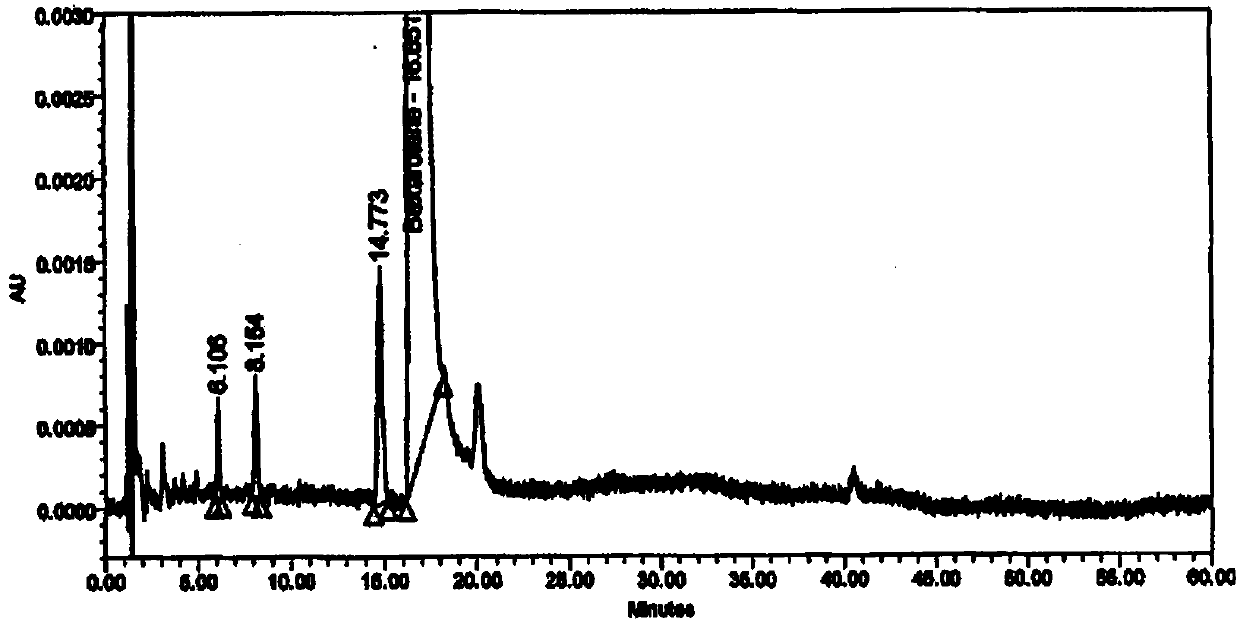
The invention provides a method for detecting impurity in bexarotene softgel by high performance liquid chromatography. In the high performance liquid chromatography, mobile phases A and B are included; the mobile phase A includes acetonitrile and a 0.01M ammoniom acetate buffer in the proportion of 70 to 30; the mobile phase B includes acetonitrile and a 0.01M ammoniom acetate buffer in the proportion of 80 to 20; and the pH value of the ammoniom acetate buffer is 3.0. Via the detection method, the impurity in the bexarotene softgel can be detected simply and rapidly, different peaks can be separated effectively, and the detection method is also simple in operation and shorter in analysis time.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Drug for retinal degenerative disease associated with photoreceptor degeneration
PendingUS20200330415A1Increase the number ofSymptoms improvedCompounds screening/testingSenses disorderAcitretinPharmacology
An object of the present invention is to provide a medicine that can simply treat and / or prevent a retinal degenerative disease associated with photoreceptor degeneration, including retinitis pigmentosa. The solution is to provide an agent for treating and / or preventing a retinal degenerative disease associated with photoreceptor degeneration, containing a compound having a retinoic acid receptor agonistic activity (for example, tamibarotene, tamibarotene methyl ester, tamibarotene ethyl ester, tazarotene, tazarotenic acid, adapalene, palovarotene, retinol, isotretinoin, alitretinoin, etretinate, acitretin or bexarotene) or a salt thereof.
Owner:DAIICHI SANKYO CO LTD
Bexarotene conjugate, drug composition and application thereof
InactiveCN106667985AEasy to degradeGood biocompatibilityOrganic active ingredientsAntimycoticsDiseaseNanoparticle
The invention discloses a bexarotene conjugate, a drug composition and application thereof. The drug composition comprises the bexarotene conjugate or a medicinal composition of the bexarotene conjugate and a pharmacodynamics acceptable carrier and is a liquid preparation, a solid preparation, a semi-solid preparation, a capsule preparation, a granule preparation, a gel preparation and an injection preparation. The drug composition is nano granules or lipidosome prepared by the bexarotene conjugate or the bexarotene conjugate and additives, and the particle size is 10 to 1000 nm. The bexarotene conjugate and the nano granules thereof can be used as liquid preparations, solid preparations, semi-solid preparations, sterilized preparations and sterile preparations, are low in toxicity and can be used for treating tumors and treating or preventing alzheimer diseases.
Owner:SOUTHEAST UNIV
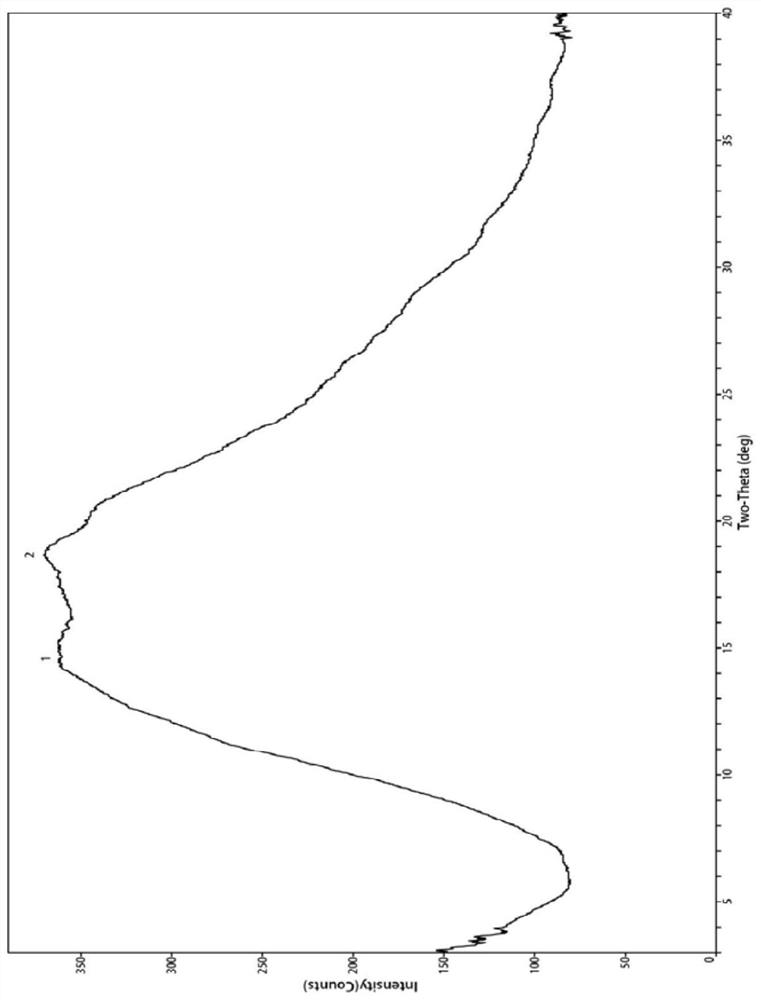
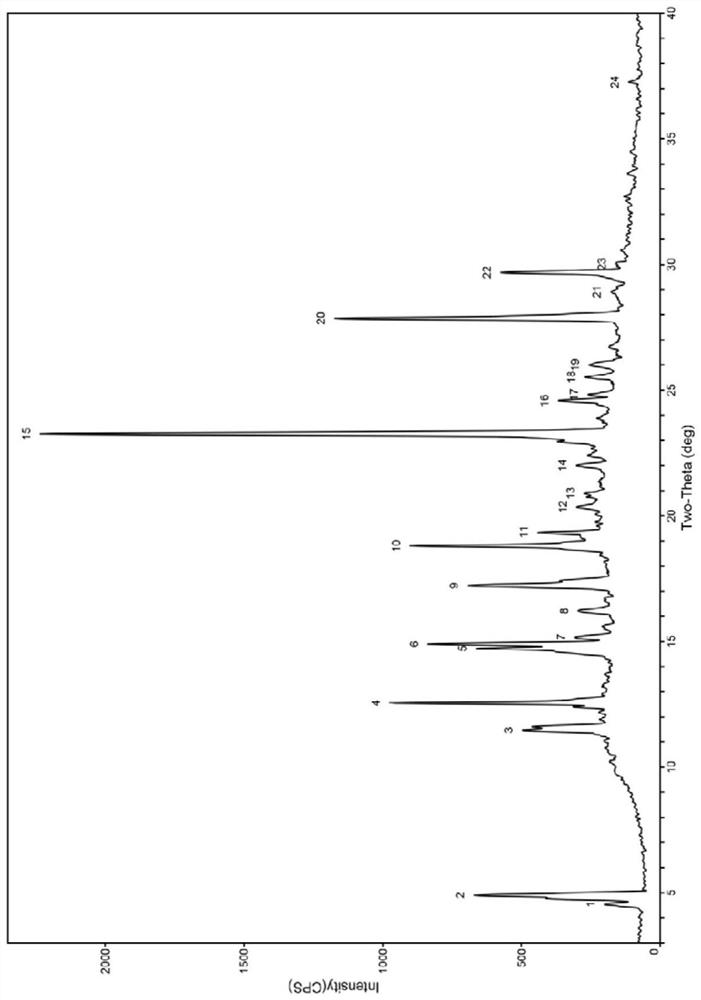
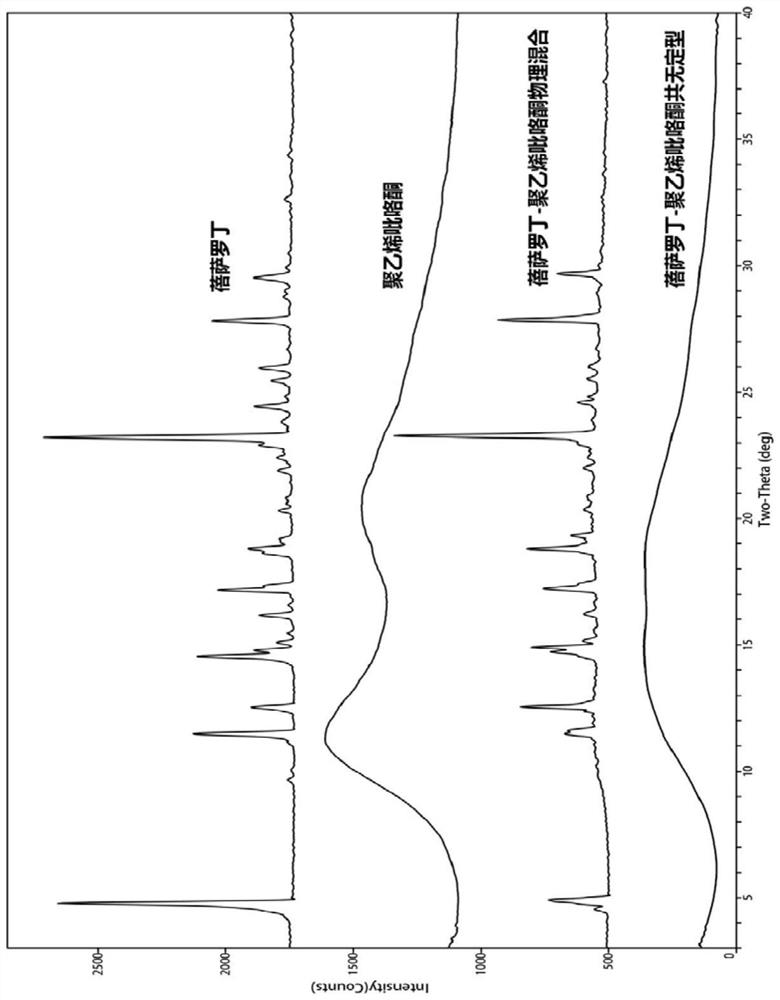
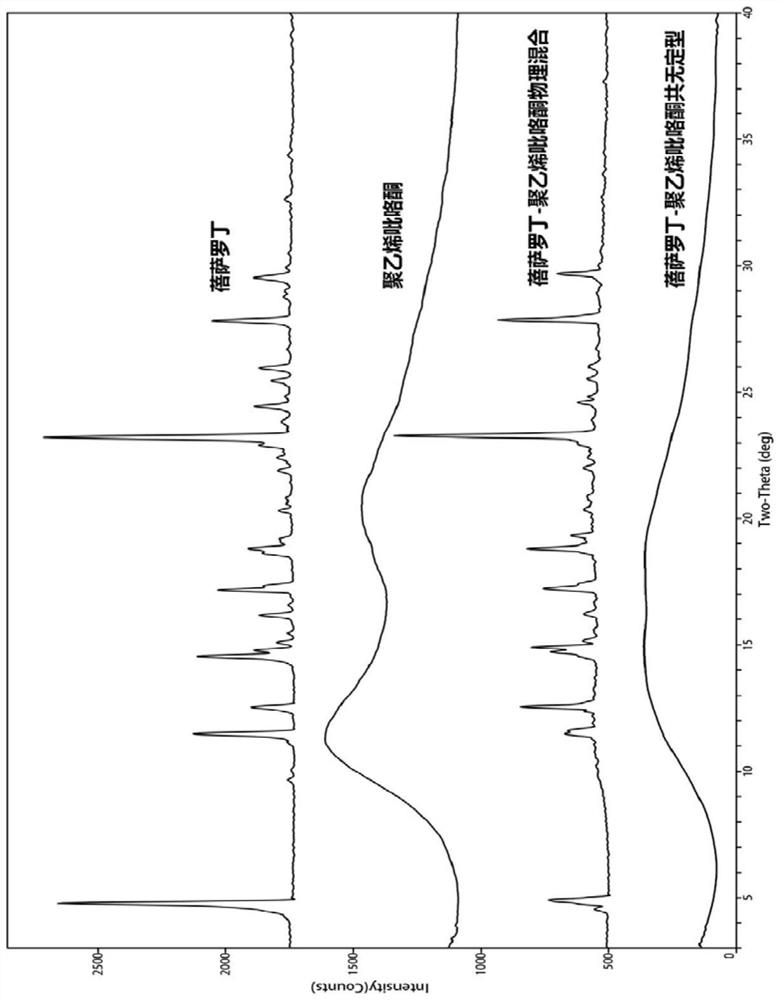
Bexarotene and polyvinylpyrrolidone co-amorphous substance, and preparation method, composition and application thereof
ActiveCN111718258AAdvantages of good safety medicineSignificant progressOrganic chemistry methodsSynthetic polymeric active ingredientsPyrrolidinonesPharmacology
The invention discloses a bexarotene and polyvinylpyrrolidone co-amorphous substance as well as a preparation method, a composition and application thereof. Specifically, the invention discloses a newco-amorphous form formed by bexarotene and polyvinylpyrrolidone. The invention discloses a method for preparing a bexarotene and polyvinylpyrrolidone co-amorphous form. The invention discloses application of bexarotene and polyvinylpyrrolidone co-amorphous form serving as an active pharmaceutical ingredient in the preparation of drugs for preventing and treating glioma, skin T-cell lymphoma, breast cancer, psoriasis, Kaposi's sarcoma, lung cancer and Cushing's disease.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Topical pharmaceutical compositions comprising bexarotene and corticosteroids
Topical pharmaceutical compositions are described comprising: a) bexarotene, b) a corticosteroid, and c) a carrier or vehicle. Said compositions are useful for the treatment of skin disorders.
Owner:ALMIRALL
Cancer stem cell proliferation inhibitor
PendingUS20200061007A1Cancer stem cells can be reducedSize of tumor is reduced and decreasedHydroxy compound active ingredientsAntineoplastic agentsPharmaceutical drugAgonist
The present invention aims to provide a growth inhibitor for cancer stem cells resistant to existing anticancer drug therapies, which growth inhibitor acts on the cells through growth inhibition and apoptosis. The growth inhibitor for cancer stem cells contains a retinoid agonist, preferably tamibarotene, alone or in combination with a rexinoid agonist, preferably bexarotene, as an effective component(s). The growth inhibitor for cancer stem cells enhances the effects of various anticancer drugs when the growth inhibitor is used in combination with the anticancer drugs.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Beisalutin sustained-release implantation agent for curing entity tumour
InactiveCN101176709AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a bexarotene sustained-release implant capability of curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises bexarotene, sustained-release excipient and a certain amount of sustained-release regulator; the amount of the bexarotene and sustained-release excipient is sufficient to control cancer; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the bexarotene can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of bexarotene can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor, and the therapeutic effects of non-operative treatments such as chemotherapeutic drugs and radiotherapy can be reinforced.
Owner:SHANDONG LANJIN PHARMA
Bexarotene derivatives and their use in treating cancer
PendingUS20210363093A1Increasing peripheral blood countImproving immune system functionOrganic active ingredientsOrganic chemistryDiseaseAutoimmune disease
This disclosure relates to compositions and methods for treating cancer. Specifically, this disclosure relates to bexarotene derivatives, methods for treating cancer, autoimmune disorders, and / or skin dermatitis, and / or methods for increasing peripheral blood counts and / or improving immune system function.
Owner:DJ THERAPEUTICS LLC
Bexarotene and polyvinylpyrrolidone co-amorphous substance, preparation method, composition and use thereof
ActiveCN111718258BAdvantages of good safety medicineSignificant progressOrganic chemistry methodsSynthetic polymeric active ingredientsPyrrolidinonesBULK ACTIVE INGREDIENT
The invention discloses a co-amorphous product of bexarotene and polyvinylpyrrolidone, a preparation method, a composition and an application thereof. Specifically, the present invention discloses a new co-amorphous form formed by bexarodene and polyvinylpyrrolidone; a preparation method of co-amorphous form of bexarotene and polyvinylpyrrolidone; Application of the amorphous form as an active ingredient of a drug in the preparation of drugs for the prevention and treatment of glioma, skin T-cell lymphoma, breast cancer, psoriasis, Kaposi's sarcoma, lung cancer and Cushing's disease.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Methods and compositions of predicting activity of retinoid X receptor modulator
The present invention describes genomic biomarkers that have been discovered to correlate with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic retinoid X receptor modulator, such as bexarotene, in treating diseases such as, non small cell lung cancer. The newly discovered biomarkers and others in linkage disequilibrium with them can be used in companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
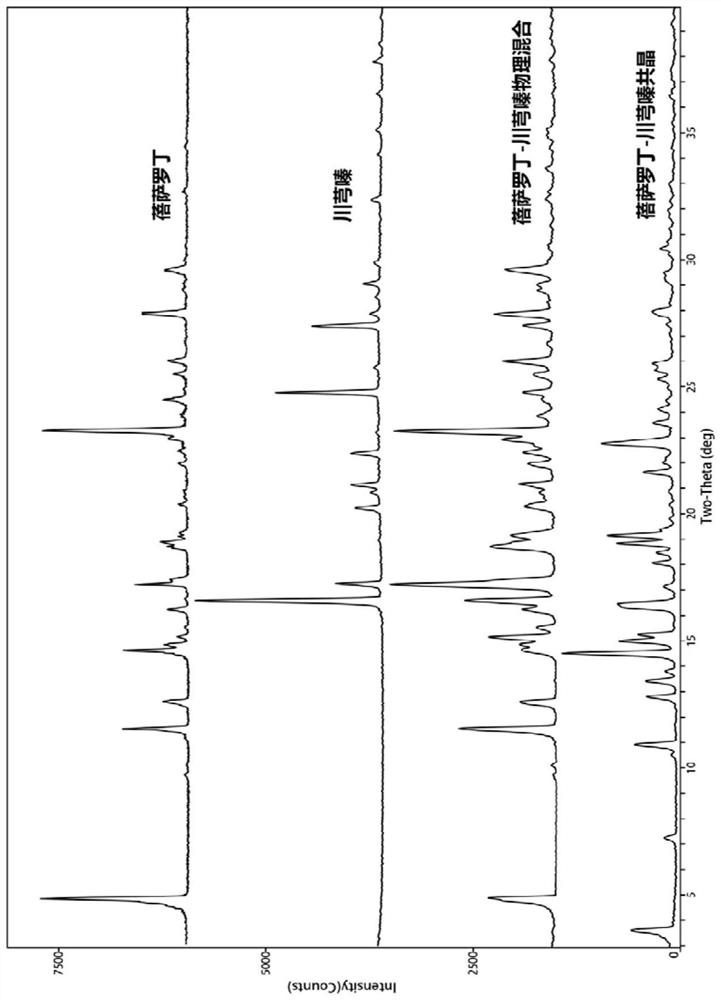
Bexarotene and ligustrazine eutectic crystal, and preparation method, composition and application thereof
ActiveCN111718257AAdvantages of good safety medicineSignificant progressOrganic active ingredientsOrganic chemistry methodsOncologyBULK ACTIVE INGREDIENT
The invention discloses a bexarotene and ligustrazine eutectic crystal as well as a preparation method, a composition and application thereof. Specifically, the invention discloses a new bexarotene and ligustrazine eutectic crystal, which takes bexarotene as a medicinal active ingredient and ligustrazine as a eutectic crystal precursor. The invention discloses a preparation method of the bexarotene and ligustrazine eutectic crystal. The bexarotene and ligustrazine eutectic crystal is used as a pharmaceutical active ingredient and is applied to preparation of drugs for preventing and treating glioma, skin T-cell lymphoma, breast cancer, psoriasis, Kaposi's sarcoma, lung cancer and Cushing's disease.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Bexarotene derivatives and their use in treating cancer
PendingCN112218629ARaise countImprove immune system functionOrganic active ingredientsOrganic chemistryOncologyPeripheral blood cell
This disclosure relates to compositions and methods for treating cancer. Specifically, this disclosure relates to bexarotene derivatives, methods for treating cancer, autoimmune disorders, and / or skindermatitis, and / or methods for increasing peripheral blood counts and / or improving immune system function.
Owner:디제이테라퓨틱스엘엘씨
Application of Bexarotene in preparation of medicine for resisting pituitary adrenocorticotropic hormone adenoma
PendingCN112999213AGrowth inhibitionLower blood ACTH levelsOrganic active ingredientsAntineoplastic agentsDiseaseAdrenal cortical hormone
The invention discloses an application of Bexarotene in preparation of a medicine for resisting pituitary adrenocorticotropic hormone adenoma, the pituitary adrenocorticotropic hormone adenoma enables a patient to excessively secrete corticotropin (ACTH) for a long time, and the adrenal gland is stimulated to secrete a large amount of cortisol, so that symptoms such as centripetal obesity, osteoporosis, amyotrophy, sexual dysfunction, hypertension, insomnia and the like of patients, which belong to rare diseases, can be caused. The Bexarotene can significantly inhibit tumor growth and inhibit ACTH secretion and expression of a precursor POMC of ACTH. The Bexarotene can be used for preparing the medicine for resisting pituitary adrenocorticotropic hormone adenoma, adverse reactions are reduced, and a safe, effective and economical solution is provided for treatment and prevention of diseases.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Application of bexarotene or/and pharmaceutically acceptable salt thereof in preparation of anti-pulmonary arterial hypertension drugs
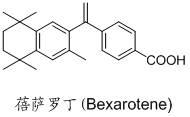
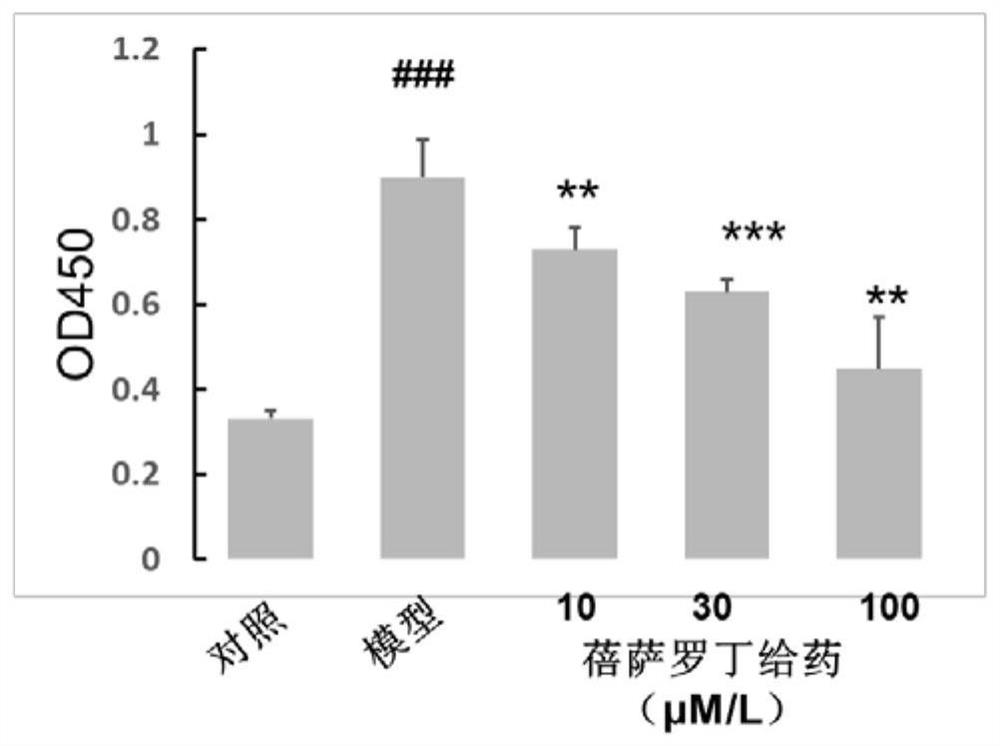
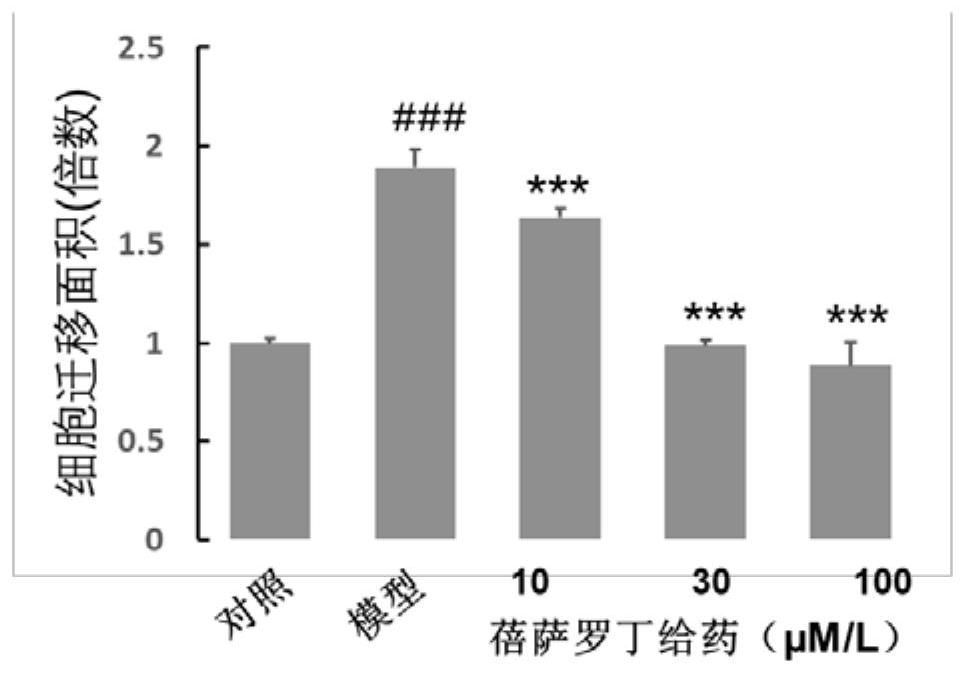
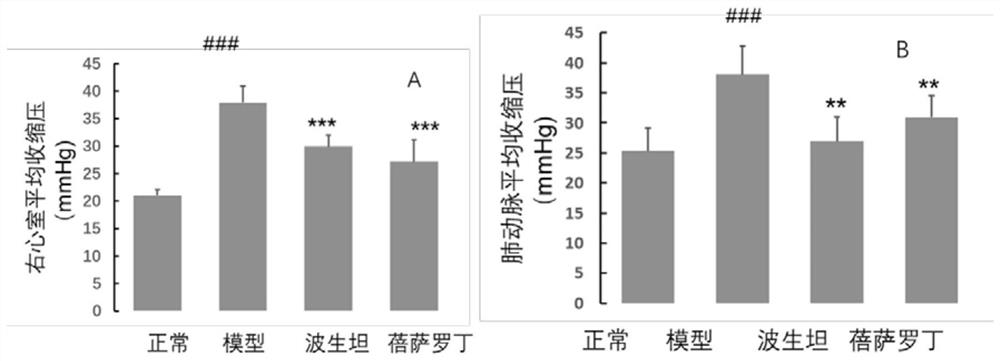
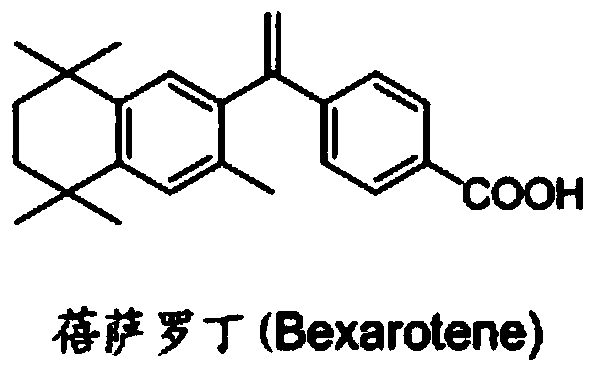
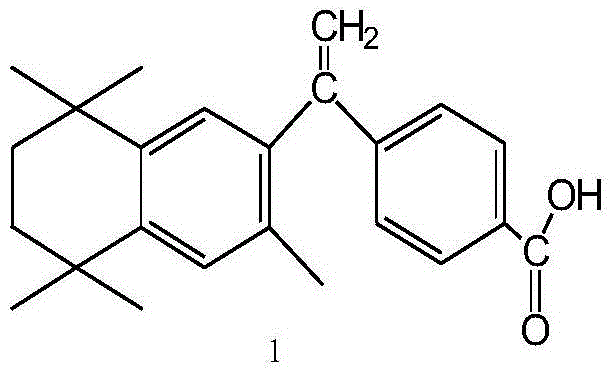
ActiveCN111956638APrevent proliferationInhibit migrationOrganic active ingredientsRespiratory disorderBenzoic acidArterial canal
The invention relates to an application of bexarotene (4-[1-(5, 6, 7, 8-tetrahydro-3, 5, 5, 8, 8-pentamethyl-2-naphthyl) vinyl] benzoic acid) or / and a pharmaceutically acceptable salt thereof in preparation of anti-pulmonary arterial hypertension drugs. The chemical structural formula of bexarotene is shown as a formula I in the specification. According to the application, rat pulmonary artery smooth muscle cells are adopted for investigating the smooth muscle cell proliferation and migration inhibiting effect of bexarotene, and the result shows that bexarotene can obviously inhibit PASMCs proliferation and migration. The monocrotaline-induced rat pulmonary arterial hypertension model is adopted to investigate the anti-pulmonary arterial hypertension vascular remodeling of the bexarotene,and the result shows that the oral administration of the bexarotene can significantly inhibit the increase of the pulmonary arterial pressure and the right ventricular systolic pressure of rats, significantly reduce the NT-proBNP level in the rat plasma, can obviously inhibit thickening of pulmonary artery intima media and reduce the narrow level of pulmonary arteriole lumens, and a safe, effective and economical solution is provided for prevention and treatment of pulmonary arterial hypertension and pulmonary vascular remodeling.
Owner:上海宛文创业孵化器管理合伙企业(有限合伙)
Compositions and Methods for Preventing Joint Destruction in Osteoarthritis
InactiveUS20130035357A1Lower Level RequirementsInhibiting degradation of collagenBiocideAntipyreticJoint destructionBexarotene
The present invention is methods for inhibiting collagen destruction in joints of osteoarthritis patients. The methods are based on use of rexinoid compounds, in particular bexarotene, and their activity to inhibit synthesis of matrix metalloproteinases (MMPs) in affected tissue.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Bexarotene hydroximic acid as well as preparation method and application thereof
InactiveCN102503857BHas anti-tumor effectEasy to prepareOrganic chemistryAntineoplastic agentsWittig reactionCancer cell
The invention belongs to the technical field of medicine and discloses bexarotene hydroximic acid as well as a preparation method and application thereof. The formation of the compound is as shown in the picture and the objective compound is prepared mainly by taking 1,1,4,4,6- pentamethyl-1,2,3,4-tetrahydronaphthalene as the initial material through the steps of Friedel-Crafts acylation, Wittig reaction and condensation reaction. The method is simple in operation, convenient in post-processing and high in yield. The objective compound has good inhibitory action on various cancer cells by the multiple action mechanisms of bonding the retinoic acid X receptors and restraining the histone deacetyltransferases, and the anti-cancer activity of the bexarotene hydroximic acid is obviously better than that of bexarotene. Therefore, the bexarotene hydroximic acid can be applied to treatment of cancers.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of key intermediate of bexarotene
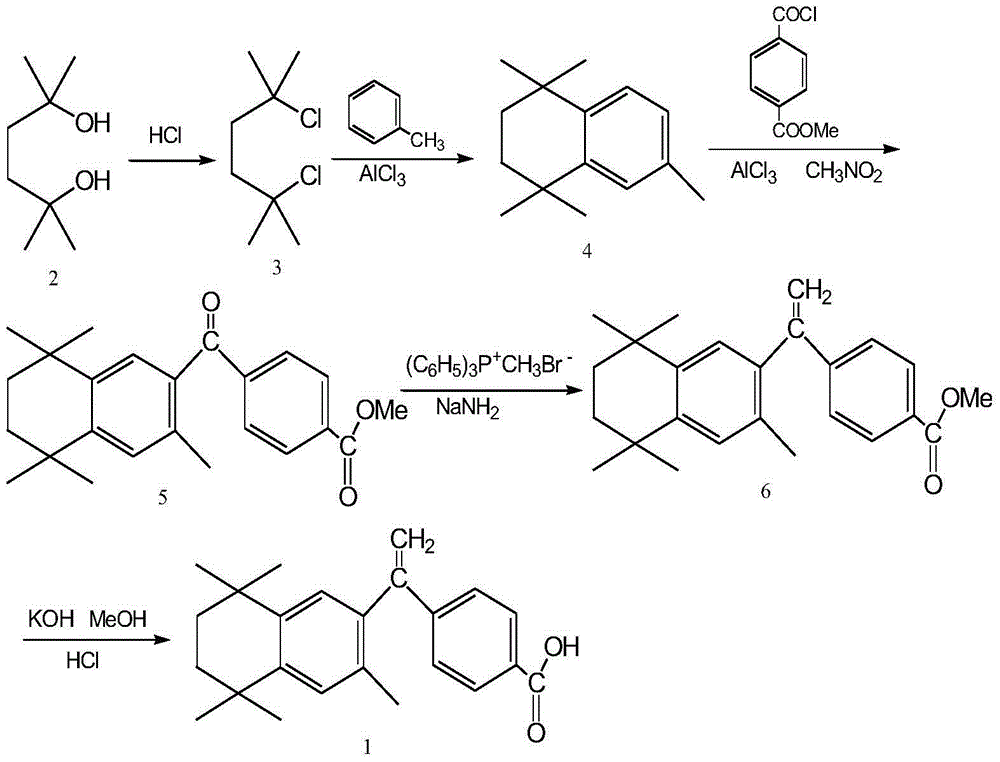
InactiveCN106146313AThe reaction steps are simpleSimple production processOrganic compound preparationCarboxylic acid esters preparationTetralinNitromethane
The invention relates to a preparation method of a key intermediate 4-[(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthalene)methoxyl]methyl benzoate (5) of new anti-cancer medicine bexarotene. The method comprises the steps that an intermediate 1,1,4,4,6-pentamethyl-1,2,3,4-tetrahydronaphthalene (4) and commercially available chemical materials of mono-methyl terephthlate, chlorinating agents of thionyl chloride or oxalyl chloride or the like and solvents of aluminum chloride, dichloromethane and the like are directly subjected to a one-step reaction, and the intermediate (5) is obtained. Please see the formula in the description. According to the preparation method of the key intermediate 4-[(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthalene)methoxyl]methyl benzoate (5) of the new anti-cancer medicine bexarotene, the reaction step is simplified, the raw materials are easy to obtain, the reaction condition is mild, the reaction yield is up to 95% (The literature method yield is 70%.), the product purity is larger than or equal to 98%, the step of preparing methoxycarbonyl benzoyl chloride through virulent phosphorus pentachloride in a literature method is omitted, a virulent and explosive chemical reagent nitromethane used for a stable reactant is removed, the production cost is reduced, the production process is simplified, and the preparation method is very suitable for industrialized mass production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Beisalutin sustained-release implantation agent for curing entity tumour
InactiveCN100563645COrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
A bexarotene slow-release implant for treating solid tumors is characterized in that the slow-release implant contains an effective anticancer dose of bexarotene, a slow-release auxiliary material and a certain amount of slow-release regulator. Solid tumors include lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostate cancer, bladder cancer, and colorectal cancer. Sustained-release excipients are mainly copolymers of glycolic acid and glycolic acid, polyphenylpropanol, poly(L-lactide-co-ethyl phosphate), and poly(L-lactide-co-propyl phosphate). One or a combination thereof can slowly release bexarotene locally in the tumor during its degradation and absorption, so while significantly reducing its systemic toxicity, it can also maintain an effective drug concentration in the local tumor. Local placement of the anti-sustained-release implant in the tumor can not only reduce the systemic toxicity of bexarotene, but also selectively increase the drug concentration in the local tumor, and enhance the therapeutic effect of non-surgical treatments such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA
Medicine composition comprising bexarotene
ActiveCN111714479AFast absorptionIncrease blood concentrationSynthetic polymeric active ingredientsDermatological disorderOncologyBULK ACTIVE INGREDIENT
The invention discloses a medicine composition comprising bexarotene, and a preparation method and a purpose of the medicine composition. Concretely, the invention discloses the medicine composition comprising the bexarotene, the preparation method of the medicine composition, and application of the medicine composition comprising the bexarotene as a medicine active ingredient to preparation of medicine for preventing and treating glioma, skin T-cell lymphoma, breast cancer, psoriasis, Kaposi's sarcoma, lung cancer and Cushing's diseases.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
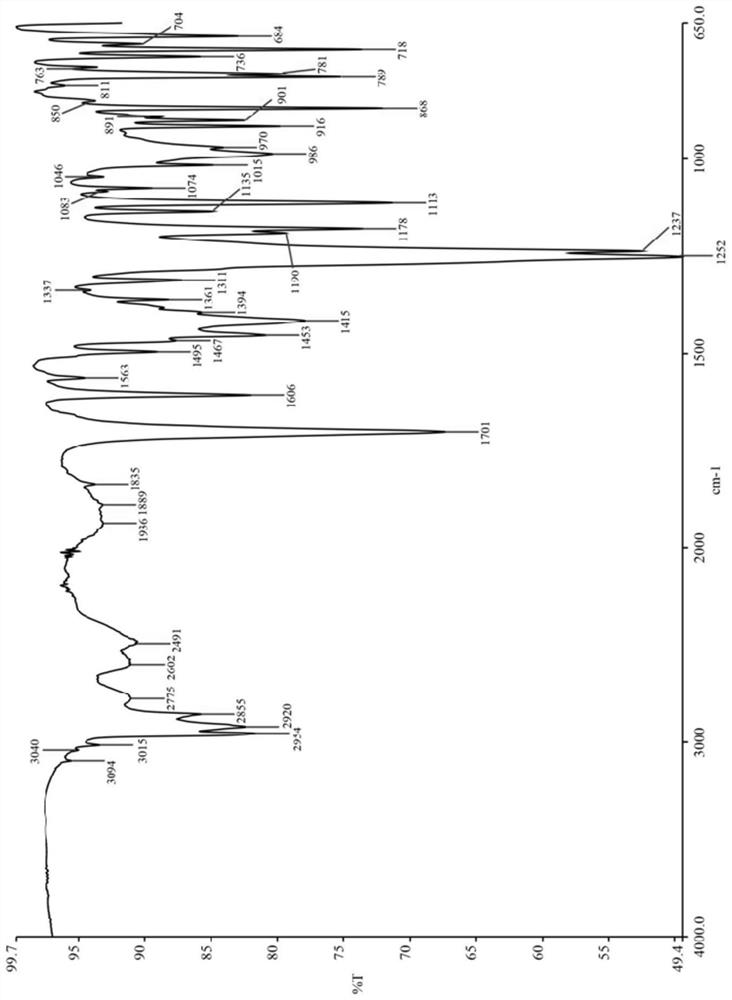
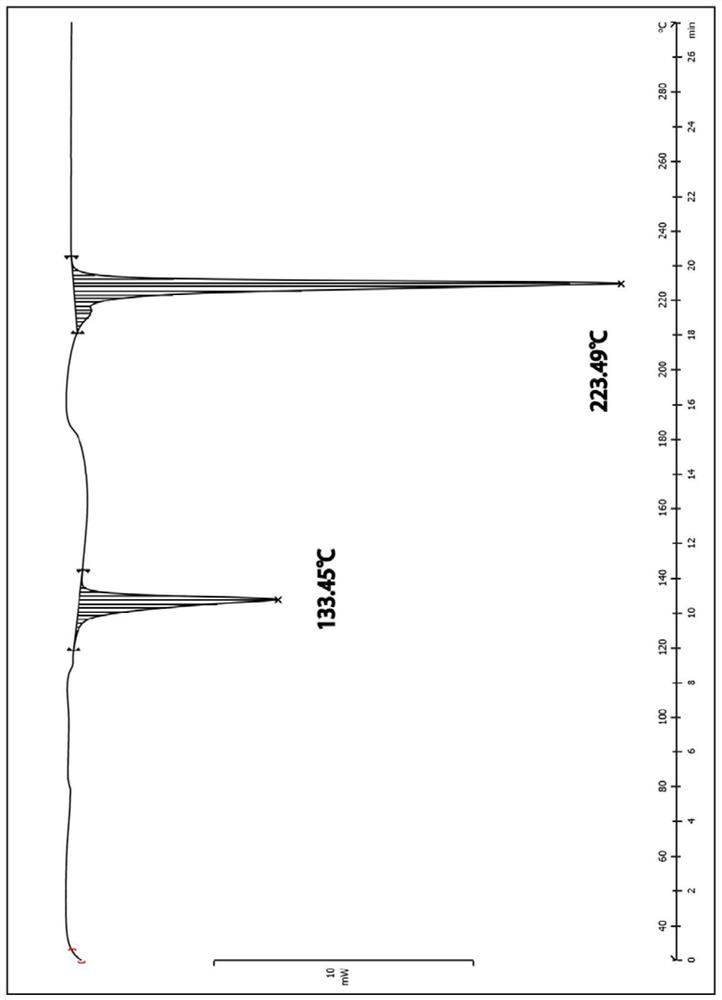
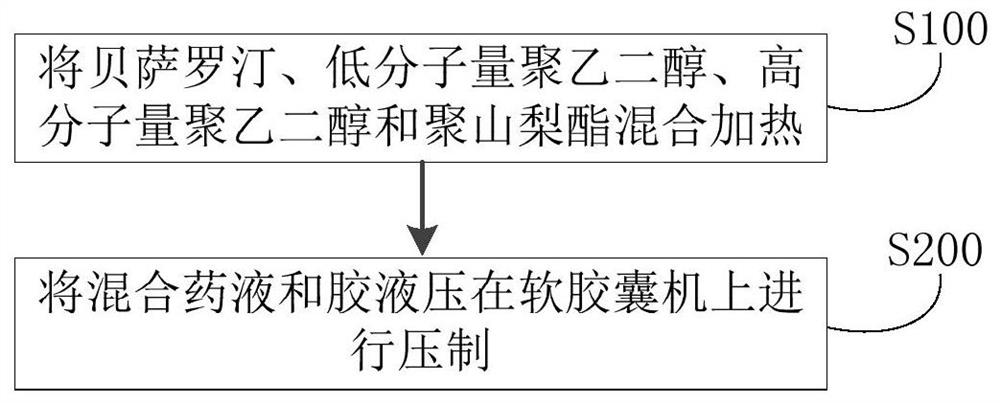
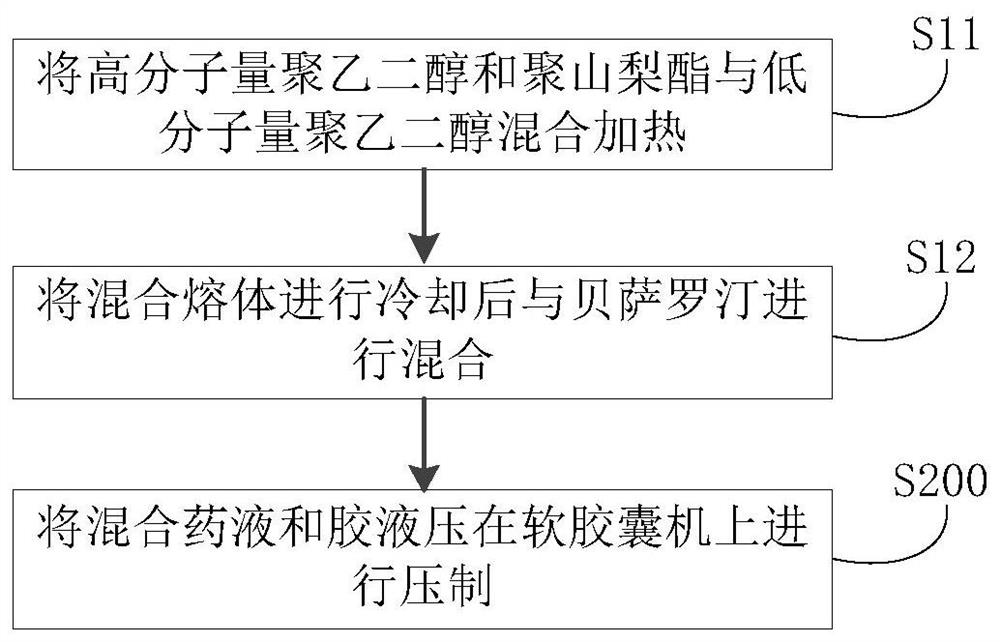
Bexarotene soft capsule and preparation method thereof
ActiveCN108434116BAvoid drug degradationAvoid degradationOrganic active ingredientsInorganic non-active ingredientsFormularySoftgel
The invention discloses a bexarotene soft capsule and a preparation method thereof. The bexarotene soft capsule comprises: contents and a soft capsule shell, wherein the contents include bexarotene, low molecular weight polyethylene Glycols, high molecular weight polyethylene glycols, and polysorbates. The bexarotene soft capsules composed of this formula can avoid drug degradation caused by high temperature exothermic and difficult degassing during the preparation of bexarotene soft capsules in the prior art while maintaining its drug release characteristics question.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
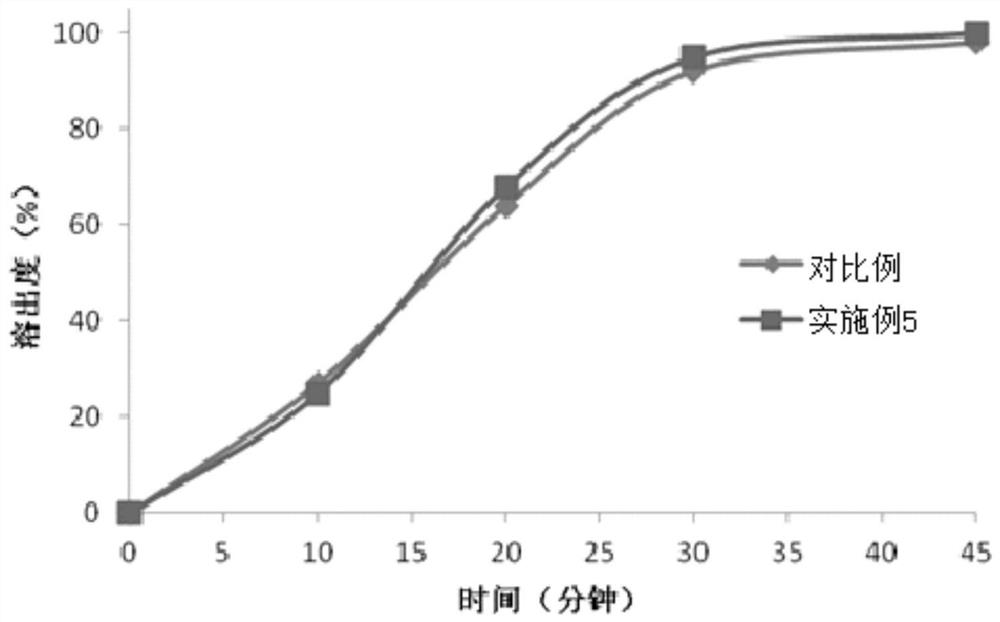
Method for determining enzyme dissolution of bexarotene softgel
ActiveCN110455932AGood test parametersObjectively reflect the actual dissolution behaviorComponent separationSoftgelDissolution
The invention discloses a method for determining enzyme dissolution of a bexarotene softgel. The method mainly comprises preparation of a dissolution medium, preparation of a tested solution, preparation of a contrast solution and determination. The method can more effectively reflect dissolution and release in the enzyme dissolution of bexarotene softgel, overcomes the problem of reduction of in-vitro release degree due to gelatin crosslinking in the capsule material, and can objectively reflect the practical dissolution and the preparation quality of the softgel.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Traditional Chinese medicine composition for deodorizing and treating dermatophytosis and deodorizing socks
InactiveCN113730505ALow costEasy to produceAntimycoticsAnthropod material medical ingredientsSmelly socksPoterium sanguisorba
The invention relates to a traditional Chinese medicine composition for deodorizing and treating dermatophytosis, which is prepared from tea leaves, pepper, garden burnet, cedar leaves, rhizoma polygonati, uniflower swisscentaury roots, white vinegar, Bexarotene, bitter, tuckahoe, alum, salt, tobacco leaves, pumpkin roots and common cnidium fruits. Natural traditional Chinese medicines are taken as raw materials, dialectical compatibility is performed, the dermatophytosis and foot odor are treated by utilizing the comprehensive action of the medicines, the medicine effect is good, meanwhile, the traditional Chinese medicine composition is low in cost and convenient to produce, socks prepared through high-temperature fumigation of the medicine composition are used for treating beriberi and preventing foot odor, the medicine odor can act on the feet for a long time through the socks, and therefore the beriberi curing period is shortened, the beriberi curing period is short, beriberi curing speed is high, meanwhile, frequent smearing of ointment is not needed, only the socks need to be worn normally, the socks are convenient to use, the feet do not need to be touched by hands, and the socks are more sanitary.
Owner:重庆市雪英服装有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com