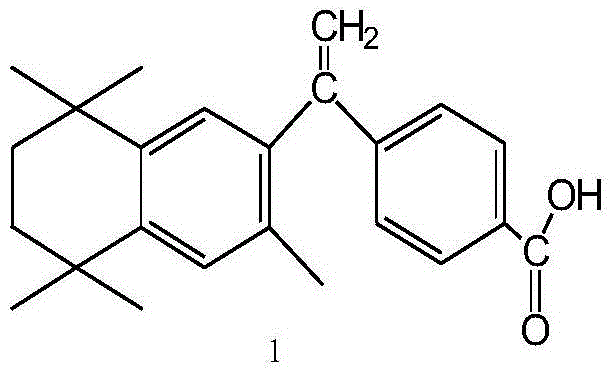
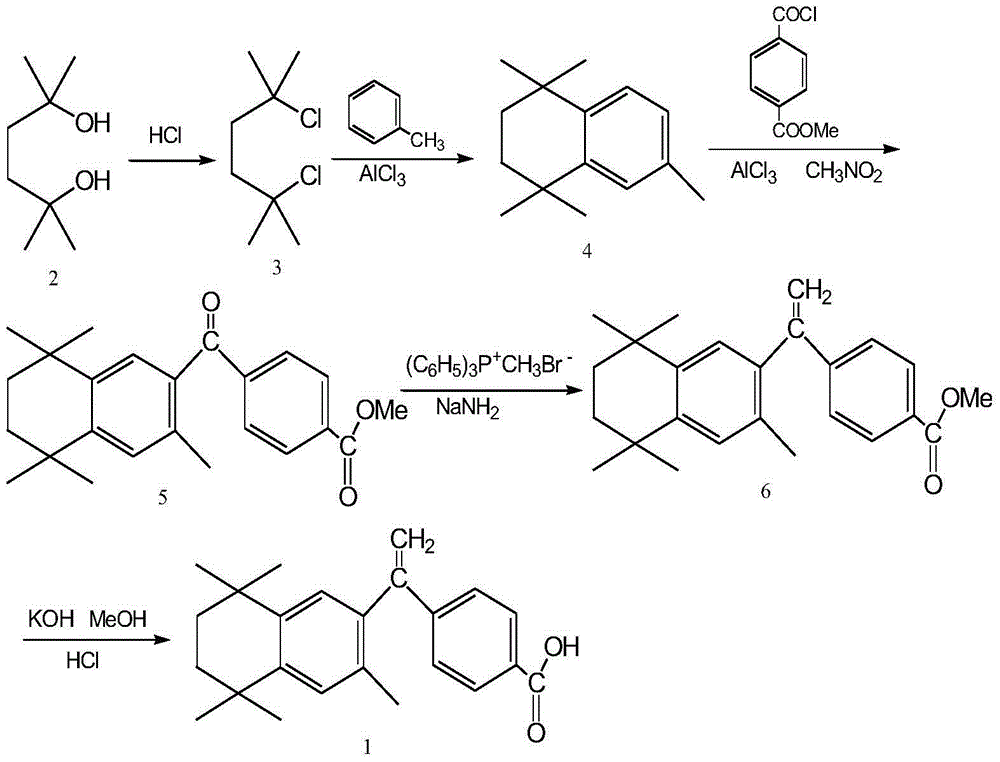
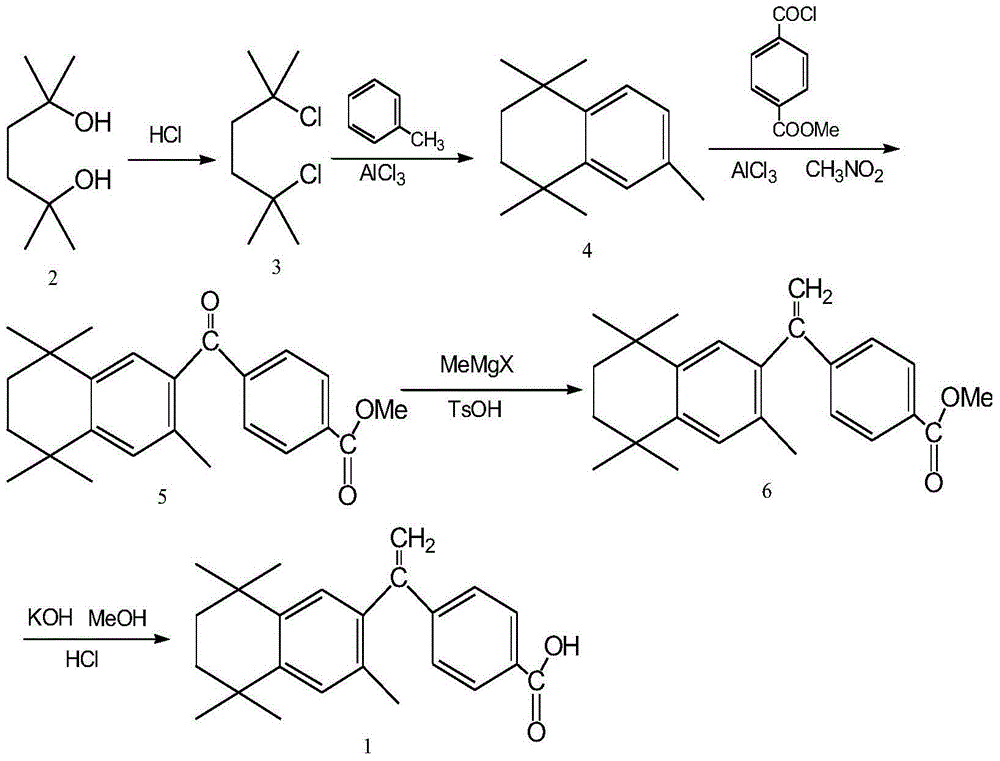
Preparation method of key intermediate of bexarotene
An intermediate and key technology are applied in the field of preparation of key intermediates of new anti-cancer drug bexarotene, can solve the problems of many side reactions, poor product 5 purity, low reaction yield and the like, simplify the production process and reduce the production Cost, effect of simplifying reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Add 120.0g (0.666mol) of monomethyl terephthalate, 58.0ml (0.799mol) of thionyl chloride, 1500ml of dichloromethane and 134.5g (0.666mol) of intermediate 4 into the reaction flask, stir well, and then add 239.6 g (1.798 mol) of aluminum trichloride was added, and the reaction was stirred at room temperature (20° C.) for 1 hour after the addition. Then heated to reflux for 3 hours, concentrated the reaction solution under reduced pressure, cooled it, slowly added ice water and stirred to dissolve, added ethyl acetate for extraction, separated the organic layer, washed twice with water, and dried over anhydrous sodium sulfate. After the organic layer was evaporated to dryness under reduced pressure, recrystallized with methanol, filtered, and dried to obtain 231.0 g of white crystalline powder, melting point: 141-143 ° C, yield 95.3% (document J.Med.Chem.1994, 37 (18): 2930~ 2941 reported melting point: 142-143°C, yield: 72%). 1 H-NMR (CDCl 3 )δ: 1.29 (6H, s, CH 3 ×2);...
example 2
[0032] Add 180.0g (0.999mol) of monomethyl terephthalate, 85.2ml (0.999mol) of oxalyl chloride, 1500ml of chloroform and 134.5g (0.666mol) of intermediate 4 into the reaction flask, stir well, and then add trichloromethane 355.2 g (2.664 mol) of aluminum chloride was added, and stirred and reacted at 30° C. for 1 hour after the addition was completed. Then heated to reflux for 5 hours, concentrated the reaction solution under reduced pressure, cooled and slowly added ice water to stir and dissolve, added ethyl acetate for extraction, separated the organic layer, washed twice with water, and dried with anhydrous sodium sulfate. After the organic layer was evaporated to dryness under reduced pressure, recrystallized with methanol, filtered, and dried to obtain 217.0 g of white crystalline powder, melting point: 141-143 ° C, yield 89.5% (document J.Med.Chem.1994, 37 (18): 2930~ 2941 reported melting point: 142-143°C, yield: 72%). 1 H-NMR (CDCl 3 )δ: 1.29 (6H, s, CH 3 ×2); 1.31...
example 3
[0034] Add 96.3g (0.533mol) of monomethyl terephthalate, 38.7ml (0.533mol) of thionyl chloride, 1500ml of toluene and 134.5g (0.666mol) of intermediate 4 into the reaction flask, stir well, and then add trichloro 88.8 g (0.666 mol) of aluminum chloride was added, and stirred and reacted at 50° C. for 1 hour after the addition was complete. Then heated to reflux for 8 hours, concentrated the reaction solution under reduced pressure, cooled and slowly added ice water and stirred to dissolve, added ethyl acetate for extraction, separated the organic layer, washed twice with water, and dried with anhydrous sodium sulfate. After the organic layer was evaporated to dryness under reduced pressure, it was recrystallized with methanol, filtered, and dried to obtain 170.4 g of white crystalline powder, melting point: 141-143 ° C, yield 70.3% (document J.Med.Chem.1994, 37 (18): 2930~ 2941 reported melting point: 142-143°C, yield: 72%). 1 H-NMR (CDCl 3)δ: 1.29 (6H, s, CH 3 ×2); 1.31 (6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com