Bexarotene conjugate, drug composition and application thereof
A technology of a composition and a conjugate, applied in the field of medicine, can solve the problems of affecting drug efficacy, obvious toxic and side effects, and less free drug content, and achieve good treatment and/or prevention of Alzheimer's disease, strong anti-tumor activity, Easy to ingest effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
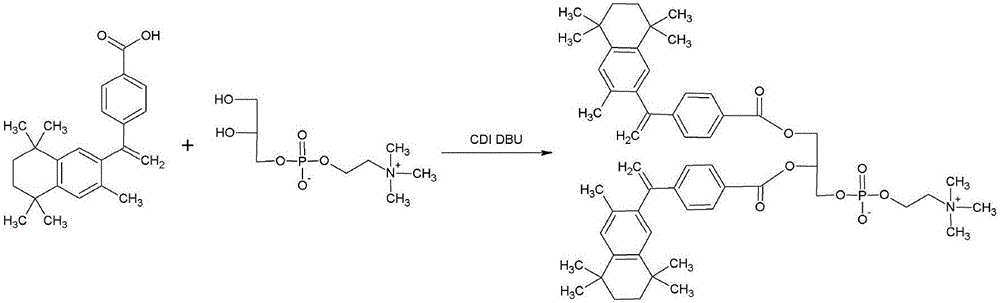
[0061] The synthesis of bexarotene glycerol phosphatidylcholine (see the synthetic route figure 1 )
[0062] Weigh 2.9 mmol of bexarotene into a 50 ml one-necked flask, add 10 ml of chloroform and stir to fully dissolve. Add 1.2mmol CDI at room temperature and mix well, react for 1-2h, TLC (thin layer chromatography) detection and follow the reaction progress. Add 1.2 mmol of GPC to the reaction system, dropwise add 2.9 mmol of DBU, stir and mix evenly. The reaction was continued for 48 h at room temperature. After TLC showed that the reaction was complete, the product was purified by column chromatography to obtain bexarotene glycerol phosphatidylcholine as a white crystalline solid with a yield of 80%.
[0063] 1 H NMR (Bruker DPX 500MHz, CD 3 OH) δ7.96 (4H, d, J = 0.40Hz), 7.35 (4H, t, J = 0.40Hz), 7.14 (4H, m, J = 0.40Hz), 5.88 (2H, dd, J = 0.40Hz ),5.64(1H,m,J=0.40Hz),5.27(2H,dd,J=0.40Hz),4.75(1H,dd,J=0.40Hz),4.62(1H,dd,J=0.40Hz), 4.26(4H,m), 3.61(2H,t,J=0.40Hz), 3...
Embodiment 2
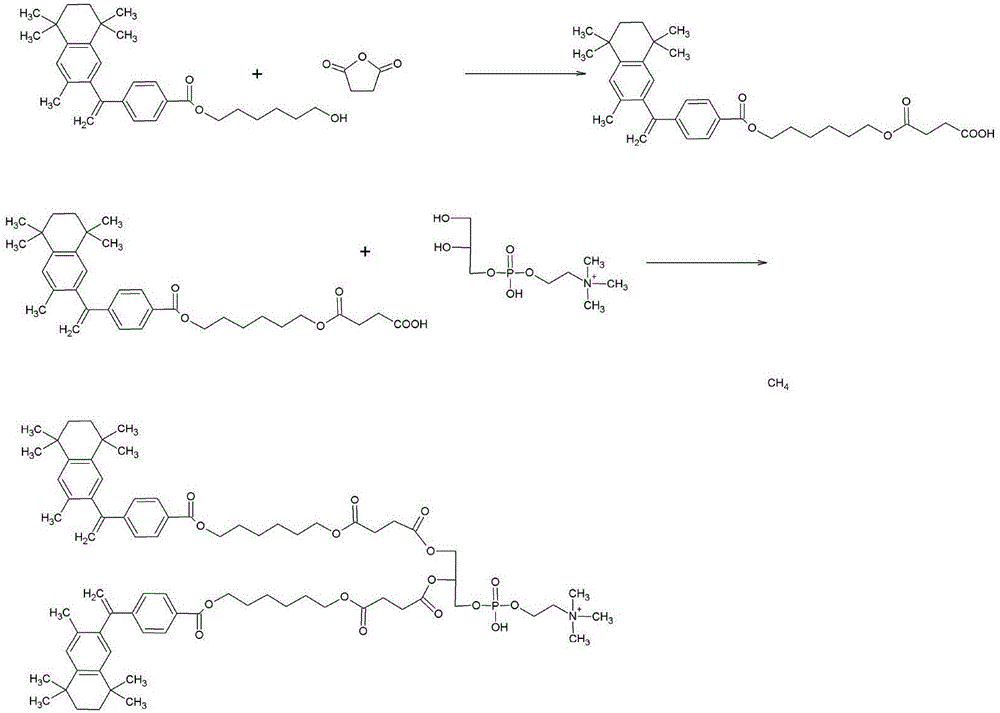
[0066] The synthesis of bexarotene hexylene glycol succinoylglycerol phosphatidylcholine (see the synthetic route figure 2 )
[0067] Weigh 1g (2.9mmol) of bexarotene into a 50ml one-necked flask, add 10ml of chloroform and stir to fully dissolve. Add 3 mmol DCC at room temperature and mix well, and react for 1-2 hours. Add 2.5 mmol hexanediol to the reaction system, drop 2.9 mmol DMAP, stir and mix evenly. The reaction was continued for 48 h at room temperature. The product was purified by column chromatography to obtain bexarotene hexanediol monoester as a white crystalline solid with a yield of 82%.
[0068] Weigh 1.6mmol bexarotene hexanediol monoester in a 100mL round bottom flask, add 20mL CH 2 Cl 2 Stir to dissolve completely. Will be fully dissolved in 10mL CH 2 Cl 2 Slowly add 10.0 mmol of succinic anhydride into the bexarotyl hexanediol monoester solution, and stir to make the reactants mix evenly. The reaction was carried out at room temperature for 3-4 ho...
Embodiment 3
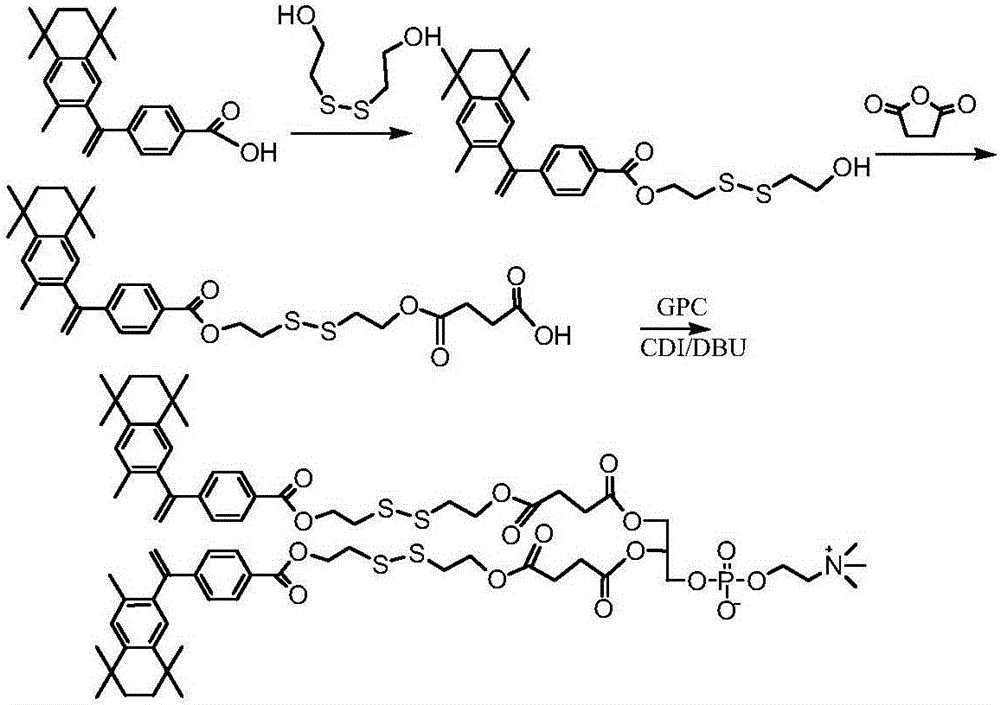
[0072] Synthesis of bexarotene dithiodiethylene glycol ester succinoylglycerol phosphatidylcholine (see the synthetic route image 3 )
[0073] Weigh 1g (2.9mmol) of bexarotene into a 50ml one-necked flask, add 10ml of chloroform and stir to fully dissolve. Add 3 mmol DCC at room temperature and mix well, and react for 1-2 hours. Add 2.5 mmol of dithiodiethylene glycol into the reaction system, drop 2.9 mmol of DMAP, stir and mix evenly. The reaction was continued for 48 h at room temperature. The product was purified by column chromatography to obtain bexarotene hexanediol monoester as a white crystalline solid with a yield of 75%.
[0074] Weigh 1.5mmol of bexarotene dithiodiethylene glycol monoester into a 100mL round bottom flask, add 20mL CH2Cl2 and stir to dissolve it completely. Slowly add 10.0 mmol succinic anhydride fully dissolved in 10 mL CH2Cl2 to the bexarotene dithiodiethylene glycol monoester solution, and stir to mix the reactants evenly. React at room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com