Bexarotene soft capsule and preparation method thereof
A soft capsule and soft capsule shell technology, applied in the field of medicine, can solve the problems of poor fluidity, heat generation, long mixing time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
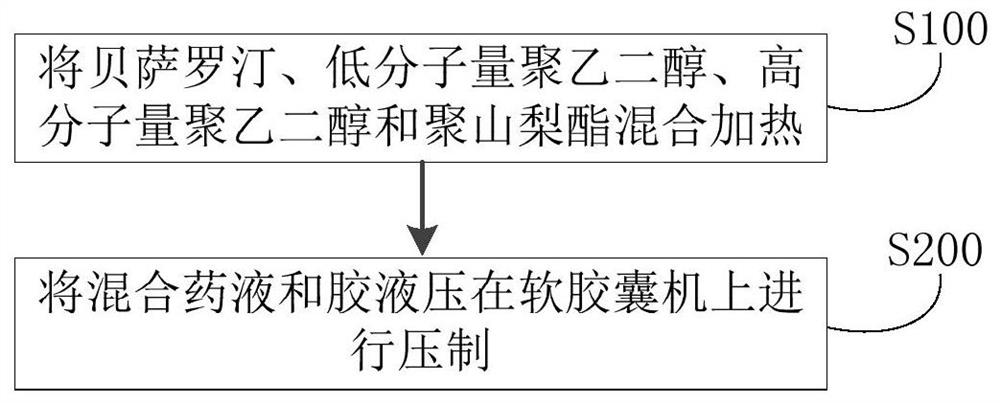
[0058] (1) Contents composition: bexarotene 75g (D90 less than 20 microns), polyethylene glycol 400 450g, polyethylene glycol 2000 75g, polysorbate 20 60g.
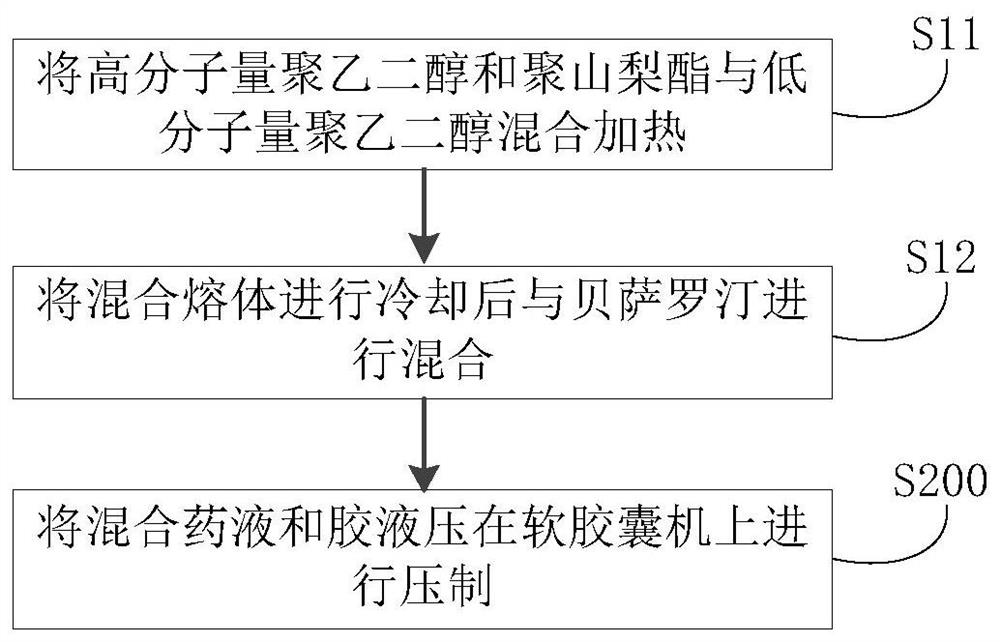
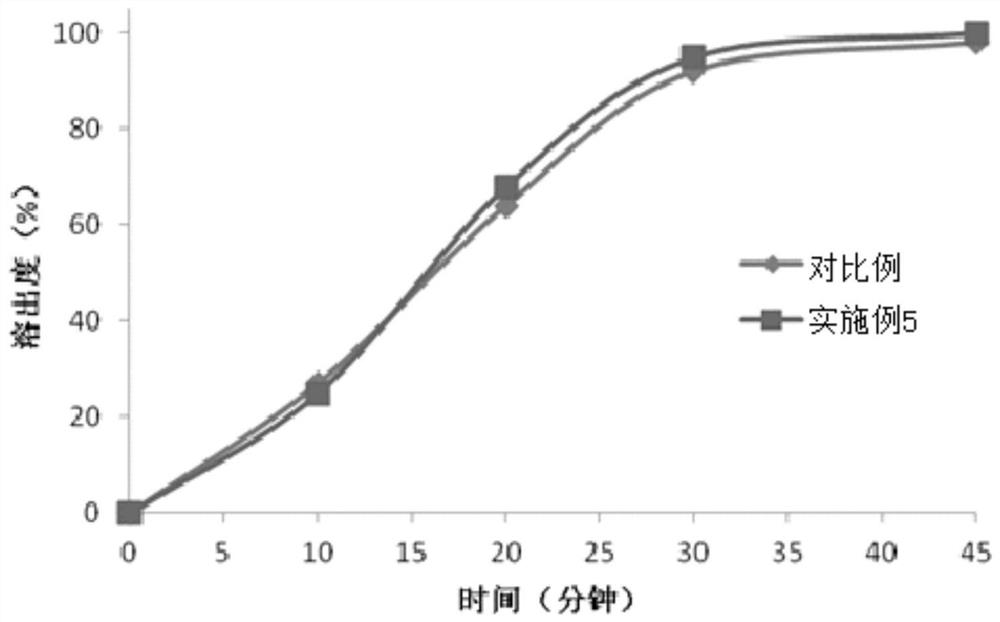
[0059] (2) Preparation method: first heat polyethylene glycol 400 to 60-80 degrees Celsius, then add polyethylene glycol 2000 and polysorbate 20 to it, stir, cool to 40-45 degrees Celsius after dissolving, and then add Bexarotene was added and stirred evenly to obtain a mixed medicinal liquid. Next, the mixed medicinal liquid is kept at 30-35 degrees Celsius, and then pressed with a glue liquid (composed of gelatin, glycerol, sorbitol, water and titanium dioxide) on a soft capsule machine, dried, polished and packaged to obtain bexarotene soft capsules. capsule. The production process data are shown in Table 1.
Embodiment 2
[0061] (1) Content composition: bexarotene 75g (D90 less than 20 microns), polyethylene glycol 600-600g, polyethylene glycol 1000-75g, polysorbate 40-45g.
[0062] (2) Preparation method: first heat polyethylene glycol 600 to 60-80 degrees Celsius, then add polyethylene glycol 1000 and polysorbate 40 to it, stir, cool down to 40-45 degrees Celsius after dissolving, and then put inside Add Bexarotene and mix well. The mixed medicinal liquid is kept at 30-35 degrees Celsius, and then compressed on a soft capsule machine with a glue liquid (composed of gelatin, glycerol, sorbitol, water and titanium dioxide), dried, polished and packaged to obtain bexarotene soft capsules . The production process data are shown in Table 1.
Embodiment 3
[0064] (1) Content composition: Bexarotene 75g (D90 less than 20 microns), polyethylene glycol 400 300g, polyethylene glycol 1000 37.5g, polysorbate 40 75g.
[0065] (2) Preparation method: first heat polyethylene glycol 400 to 60-80 degrees Celsius, then add polyethylene glycol 1000 and polysorbate 40 to it, stir, cool down to 40-45 degrees Celsius after dissolving, and then put inside Add Bexarotene and mix well. The mixed medicinal liquid is kept at 30-35 degrees Celsius, and then compressed on a soft capsule machine with a glue liquid (composed of gelatin, glycerol, sorbitol, water and titanium dioxide), dried, polished and packaged to obtain bexarotene soft capsules . The production process data are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com