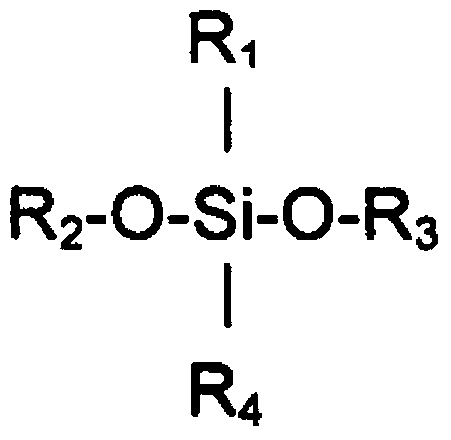Topical pharmaceutical compositions comprising bexarotene and corticosteroids
A kind of technology of corticosteroid, topical medicine, is applied in the field of preparing described composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
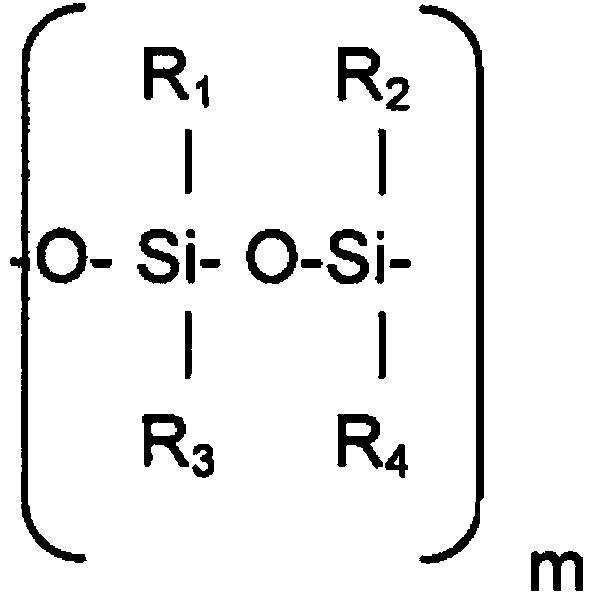
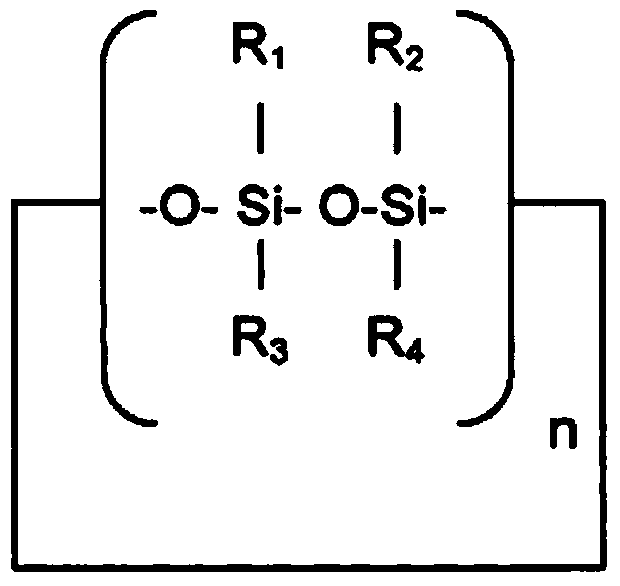
[0129] Specific examples include cyclic methylsiloxanes, which have the formula [(CH 3 ) 2 SiO] x , where x is 3-6, or a short-chain linear methylsiloxane having the formula ((CH 3 ) 2 SiO[(CH 3 ) 2 SiO] y Si(CH 3 ) 3 , where y is 0-5.
[0130] Some suitable cyclomethicones are hexamethylcyclotrisiloxane (D3), a boiling point of 134°C and of the formula [(Me 2 )SiO] 3 The solid; octamethylcyclotetrasiloxane (D4), the boiling point is 176 ° C, the viscosity is 2.3mm 2 / s and is the formula [(Me 2 )SiO] 4 ; Decamethylcyclopentasiloxane (D5) (cyclomethicone), with a boiling point of 210°C and a viscosity of 3.87mm 2 / s and is the formula [(Me 2 )SiO] 5 ; and dodecamethylcyclohexasiloxane (D6), with a boiling point of 245°C and a viscosity of 6.62mm 2 / s and is the formula [(Me 2 )SiO] 6 .
[0131] Some suitable short linear methylsiloxanes are hexamethyldisiloxane (MM) with a boiling point of 100°C and a viscosity of 0-65mm 2 / s and is the formula Me 3 SiOMe ...
Embodiment 1-6
[0149] Preparation of compositions of the present invention as shown in Table 1 (% by weight, based on the total weight of the composition)
[0150] Table 1
[0151]
[0152] The composition is prepared in the following manner:
[0153] 1) Add octyldodecanol into a stainless steel container and heat to 80°C with stirring. Bexarotene was added and dissolved while stirring to obtain a clear solution.
[0154] 2) White soft paraffin and propylene glycol monopalmitostearate are added in a stainless steel container. The ingredients were heated to 60°C and melted with stirring. The molten mixture of lipophilic compounds is homogeneous and clear.
[0155] 3) Transfer and suspend (thoroughly disperse) betamethasone dipropionate into the molten mixture of lipophilic compounds while homogenizing.
[0156] 4) Transfer the bexarotene solution to the batch while stirring.
[0157] 5) Dissolve anhydrous citric acid and sodium citrate in purified water and transfer this solution to ...
Embodiment 7-9
[0161] Preparation of compositions of the present invention as shown in Table 2 (% by weight, based on the total weight of the composition)
[0162] Table 2
[0163]
[0164]
[0165] The composition is prepared in the following manner:
[0166] 1) Add liquid paraffin, white soft paraffin and propylene glycol monopalmitostearate into a stainless steel container. The ingredients were heated to 60°C and melted with stirring. The molten mixture of lipophilic compounds is homogeneous and clear.
[0167] 2) Transfer bexarotene and betamethasone dipropionate into the molten mixture of lipophilic compounds and suspend (thoroughly disperse) while homogenizing.
[0168] 3) Dissolve anhydrous citric acid and sodium citrate in purified water and transfer this solution to the batch while homogenizing.
[0169] 4) Cool the batch to 30°C while stirring.
[0170] 5) The batch was then filled directly into glass bottles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com