Vitamin A compound and paeonol condensed derivatives and preparation method
A technology of paeonol and derivatives is applied in the field of derivatives and preparations of the condensation of vitamin A compounds and paeonol, and can solve the problems of reducing skin and mucous membrane irritation of vitamin A compounds, so as to promote epithelial cell differentiation, lipid Increased solubility and reduced irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
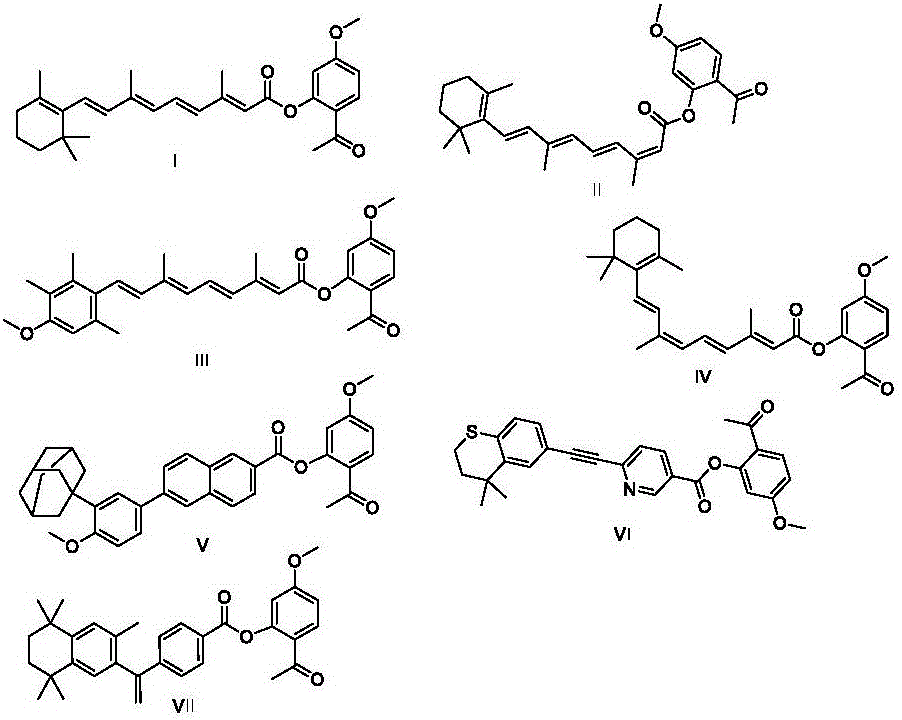
[0048] One-step method, using one-step method to synthesize compound I((2E,4E,6E,8E)-2-acetyl-5-methoxyphenyl3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl )nona-2,4,6,8-tetraenoate).
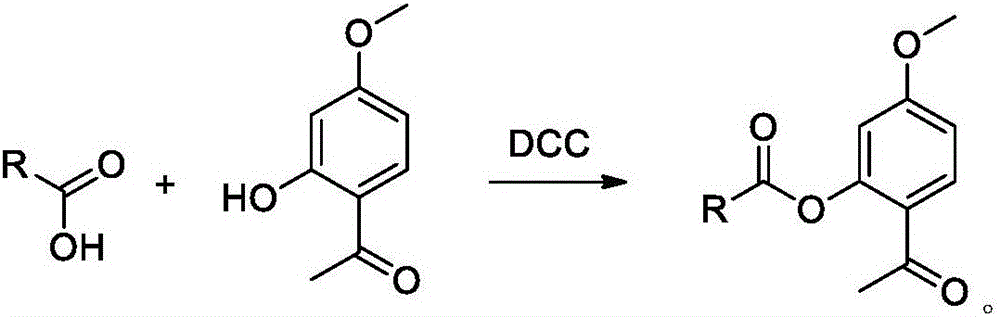
[0049] In a 250mL round-bottom flask, add 100mL of dry dichloromethane, 10.0g of tretinoin, 5.56g of paeonol, 6.87g of DCC and 0.21g of DMAP, and stir for 24h under nitrogen at room temperature and protected from light;
[0050] The solid was removed by filtration, the filtrate was concentrated, and the obtained oily product was purified by silica gel column chromatography, and the column chromatography eluent was hexane-ethyl acetate (100:1 to 5:1);
[0051] The corresponding effluent was collected and concentrated and dried with a rotary evaporator to obtain a yellow solid. LCMS: 449.4 (M+1). mp: 101-102°C. H NMR (Brucker AV-300, CDCl3, δppm): 1.04 (s, 6H), 1.46-1.49 (m, 2H), 1.59-1.64 (m, 2H), 1.73 (s, 3H), 2.03 (brs, 5H) ),2.40(s,3H),2.52(s,3H), 3.83(s,3H), 6.02(s,1H), 6.15–6.41(m,4H),6.91(...
Embodiment 2
[0054] Synthesis of compound I using a two-step method
[0055] ((2E,4E,6E,8E)-2-acetyl-5-methoxyphenyl3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6, 8-tetraenoate).
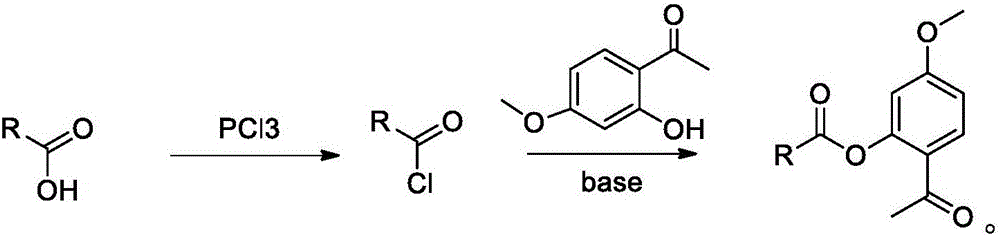
[0056] In a 250mL round bottom flask, add 70mL of dry dichloromethane and 10.0g of tretinoin;
[0057] Cool with ice water to an internal temperature of 5°C, slowly add 4.2 g of oxalyl chloride dropwise, and stir in an ice water bath for 2 hours after the addition is complete;
[0058] 5.56g of paeonol and 6.72g of triethylamine were dissolved in 50mL of dry dichloromethane and slowly added dropwise;
[0059] After the addition is complete, remove the ice-water bath, stir at room temperature for 2 hours, add 50 mL of water, and separate;
[0060] Wash the organic phase three times with 50mL each time;
[0061] Na for organic phase 2 SO 4 After drying and concentration, the obtained oily product was purified by silica gel column chromatography. The column chromatography eluent is hexane-ethyl acetate (100:1 ...
Embodiment 3
[0065] The configuration of vitamin A compound solution. Compound I (10.0g) was dissolved in isosorbide dimethyl ether (90.0g) to obtain a 10% solution, which should be stored away from light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com