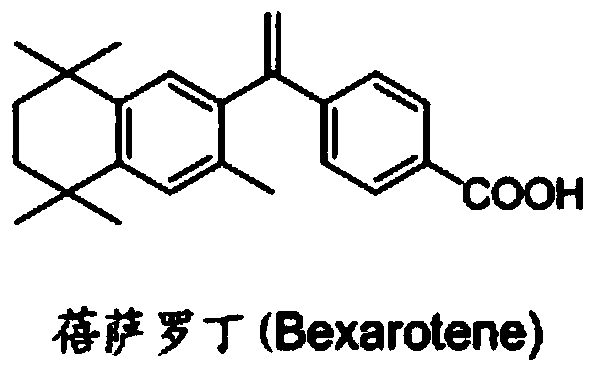
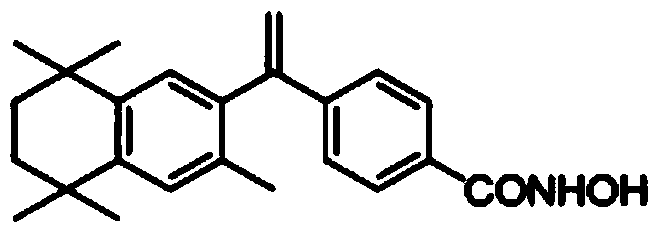
Bexarotene hydroximic acid as well as preparation method and application thereof
A technology of bexarotene hydroxamic acid and bexarotene hydroxamic acid, which is applied in the field of medicine, can solve the problems of molecular expression imbalance, malignant transformation of cells, etc., and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Preparation of methyl 4-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalene-2-carbonyl)benzoate (Ⅲ):
[0022] Into a 2L three-necked flask, add 100g of monomethyl terephthalate monoacyl chloride (II) and 80g of pentamethyltetrahydronaphthalene (I), then add 500mL of dichloromethane, stir to dissolve, and add 200g of trichloride Iron, then react at 30-35°C for 20 hours, slowly pour the reaction solution into 2L ice-water mixture under stirring, separate the layers, separate the dichloromethane layer, extract the water layer with 500ml×3 dichloromethane, and combine the organic layers . The combined organic layers were washed with saturated NaHCO 3 The aqueous solution was washed three times with 300ml × 3, and the saturated NaCl aqueous solution was washed once with 300ml, dried over anhydrous magnesium sulfate for 1 hour, and the solvent was evaporated to obtain a light yellow solid product with a crude yield of 95%.
Embodiment 24-
[0023] Example 2. Preparation of methyl 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)vinyl]benzoate (IV) :
[0024] Into a 500mL three-necked flask, put 50g of methyltriphenylphosphine bromide, 4.6g of sodium hydroxide and 22.4g of 4-(3,5,5,8,8-pentamethyl-5,6,7, 8-Tetralin-2-carbonyl) methyl benzoate (Ⅲ), then add 230mL THF, slowly raise the temperature and reflux for 10 hours, evaporate THF under reduced pressure to obtain 4-[1-(3,5,5,8 , 8-pentamethyl-5,6,7,8-tetrahydronaphthalene-2-yl) vinyl] methyl benzoate, the yield was 65%.
Embodiment 3
[0025] Embodiment 3. The preparation of bexarotene hydroxamic acid:
[0026] To a 500mL eggplant-shaped bottle, add 20g of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalene-2-yl)vinyl]benzoic acid methyl ester, 6g hydroxylamine hydrochloride and 9g sodium hydroxide, then add 200mL ethanol, stir and react at room temperature for 20 hours, filter, evaporate the filtrate to remove the solvent under reduced pressure to obtain the crude product of bexarotene hydroxamic acid, and recrystallize from ethanol to obtain the refined product. ESI-MS: 364.2[M+H] + , 362.1[M-H] -1 H-NMR (CDCl 3 )δ:1.28(s,6H),1.31(s,6H),1.70(s,4H),1.95(s,3H),5.35(d,1H),5.83(d,1H),7.08(s,1H ),7.13(s,1H),7.38(d,2H),8.03(d,2H)
[0027] Experimental part of biological activity test
[0028] 2.1 The target compound bexarodene hydroxamic acid is tested for the inhibitory activity of U87 cell line proliferation
[0029] 2.1.1 Purpose of the experiment
[0030] For the proposed synthesized bexarotene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com