Medicine composition comprising bexarotene
A bexarotene and composition technology, applied in the field of pharmaceutical compositions and preparations containing bexarotene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation method of pharmaceutical composition containing bexarotene 1
[0060] Weigh 50 mg of bexarotene crystal type I, 88 mg of bexarotene and ligustrazine eutectic, and 100 mg of co-amorphous bexarotene and polyvinylpyrrolidone 1:1, and physically mix them uniformly according to conventional methods. get.
[0061] Preparation method of pharmaceutical composition containing bexarotene 2
[0062] Weigh 50 mg of bexarotene crystal type I, 440 mg of bexarotene and ligustrazine eutectic, and 500 mg of co-amorphous bexarotene and polyvinylpyrrolidone 1:1, and physically mix them uniformly according to conventional methods. get.
Embodiment 2
[0064] Preparation method of bexarotene and ligustrazine cocrystal 1:
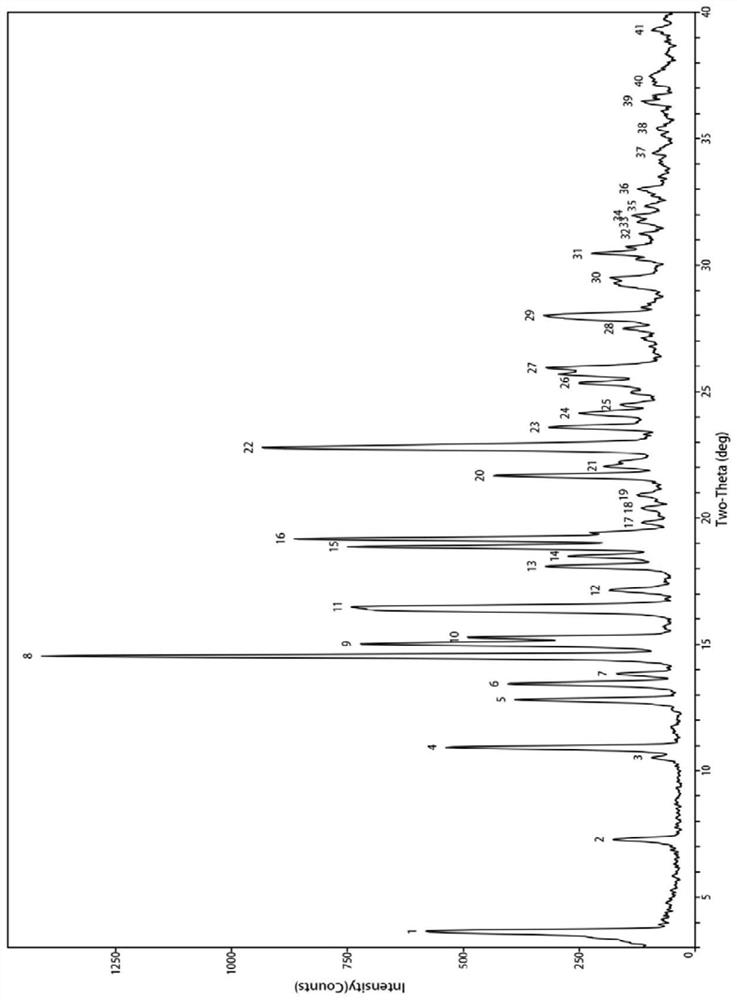
[0065] According to the table below, take appropriate amounts of bexarotene and ligustrazine into a mortar, add an appropriate amount of organic solvent, manually grind for an appropriate time, and dry at high temperature. Its powder X-ray diffraction analysis was carried out, and its diffraction pattern was the same as figure 1 Consistent, indicating that the obtained sample is a co-crystal of bexarotene and ligustrazine.
[0066]
[0067] Preparation method 2 of bexarotene and ligustrazine cocrystal:
[0068] As shown in the table below, take an appropriate amount of bexarotene and ligustrazine into a ball mill jar, add an appropriate amount of organic solvent, select an appropriate ball-to-material ratio, set an appropriate rotation speed, grind for an appropriate time, and dry at high temperature. Its powder X-ray diffraction analysis was carried out, and its diffraction pattern was the same as f...
Embodiment 3
[0074] Preparation method of bexarotene and polyvinylpyrrolidone co-amorphous product 1:
[0075] According to the table below, take an appropriate amount of bexarotene and polyvinylpyrrolidone in a mass ratio of 1:1 and put them in a ball mill. Its powder X-ray diffraction analysis was carried out, and its diffraction pattern was the same as Figure 4 Consistent, indicating that the obtained sample is a co-amorphous product of bexarotene and polyvinylpyrrolidone.
[0076]
[0077] Preparation method 2 of co-amorphous substance of bexarotene and polyvinylpyrrolidone:
[0078] Take 3 parts of Bexarotene 20g, and weigh 4g, 20g, 100g of polyvinylpyrrolidone respectively according to the mass ratio of 5:1, 1:1, 1:5, put them into the ball mill respectively, and select the ball-to-material ratio of 6: 1. The rotation speed is 400 r / min, the grinding is stopped for 2 minutes every 15 minutes, and the ball is milled for 10 hours, 2 hours, and 0.5 hours, respectively, to obtain t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com