Method for detecting impurity in bexarotene softgel by high performance liquid chromatography
A high-performance liquid chromatography and soft capsule technology, which is applied in the field of high-performance liquid chromatography for detecting impurities in besarotene soft capsules, can solve problems such as undocumented impurity detection methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of mobile phase A is: thoroughly mix 700mL acetonitrile and 300mL 0.01M ammonium acetate buffer and degas; the preparation method of mobile phase B is: thoroughly mix 800mL acetonitrile and 200mL 0.01M ammonium acetate buffer gas; the preparation method of 0.01M ammonium acetate buffer solution is: dissolve 0.77g ammonium acetate in 1L deionized water, and adjust the pH of the solution to 3.0 with glacial acetic acid.
[0038] Tests have proved that the contents of bexarotene soft capsules are insoluble in water, but soluble in more polar organic solvents, such as methanol and acetonitrile, and the capsule shell is insoluble in methanol at room temperature. Therefore, methanol was chosen as the diluent.
[0039] Preparation of reference substance stock solution: Weigh 6.0 mg of bexarotene standard substance into a 100mL volumetric flask, add diluent to dilute to the mark, and shake well to obtain a reference substance stock solution with a bexarot...
Embodiment 1
[0045] Good system adaptability:
[0046] It has been verified that the diluent does not interfere with the main peak, the theoretical plate number of the main peak is 18501, the tailing factor of the main peak is 1.1, and the RSD of the first 6 sequences against the main peak area of the standard solution is 0.2%.
Embodiment 2
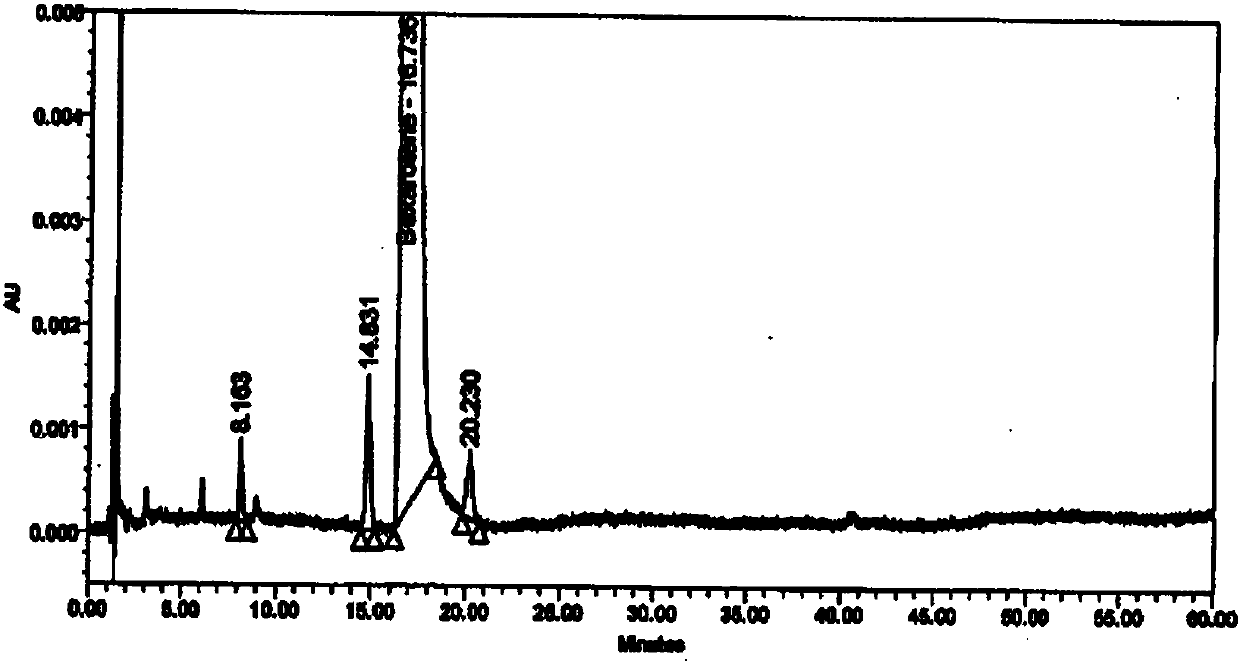
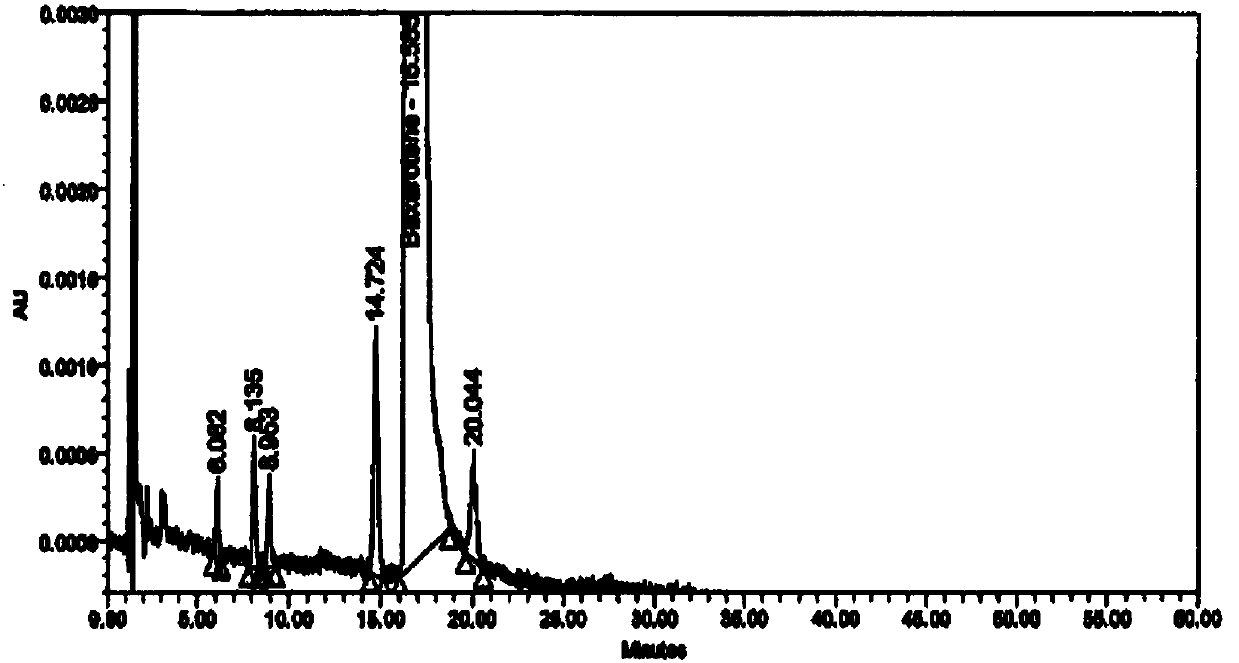
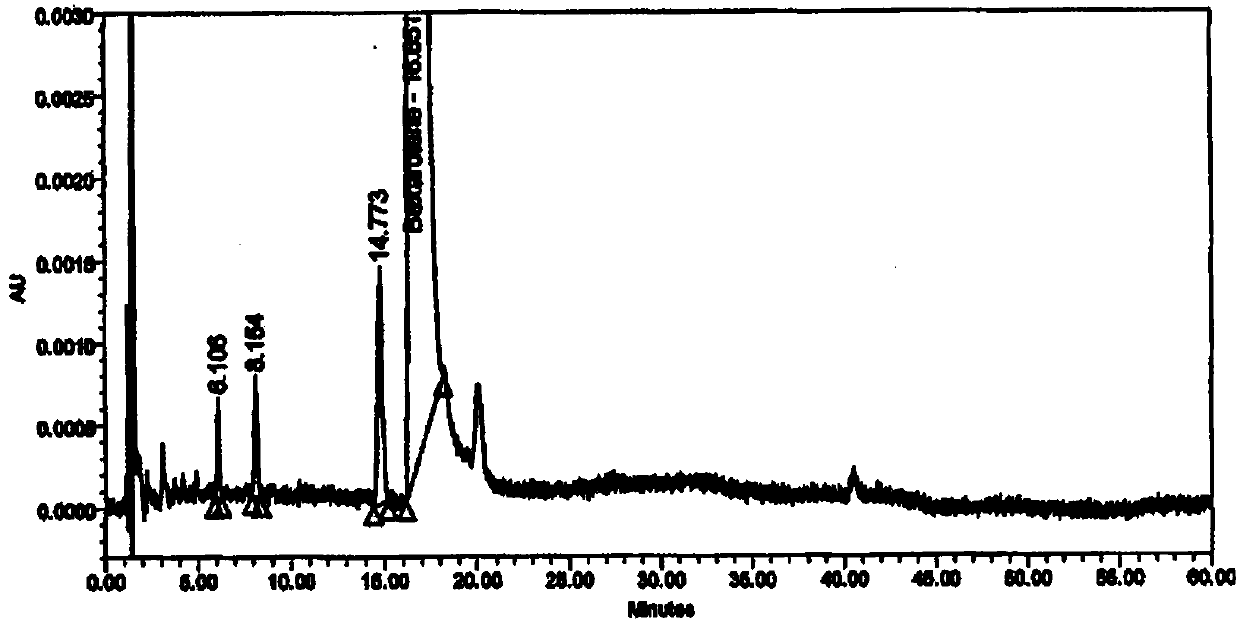
[0048] Resolution: (1) The diluent and blank excipients do not interfere with the main peak of besarotene, related substance a, related substance b, and keto acid impurity peak; (2) The resolution between the main peak and adjacent peaks is 11.5; (3) The retention times and relative retention times of the main peak and each impurity peak are shown in Table 1.
[0049] Table 1 Retention time and relative retention time of main peak and each impurity peak
[0050] name betha rotten Impurity a Impurity b Keto acid impurities Types of raw material process impurities process impurities process impurities keep time 16.82 24.25 41.05 8.30 relative retention time -- 1.4 2.4 0.5
[0051] Strong degradation test: (1) The impurity peaks produced in any strong degradation test do not interfere with the main peak, (2) The peak purity threshold of the main peak is greater than the peak purity angle of the reference substance and the degra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com