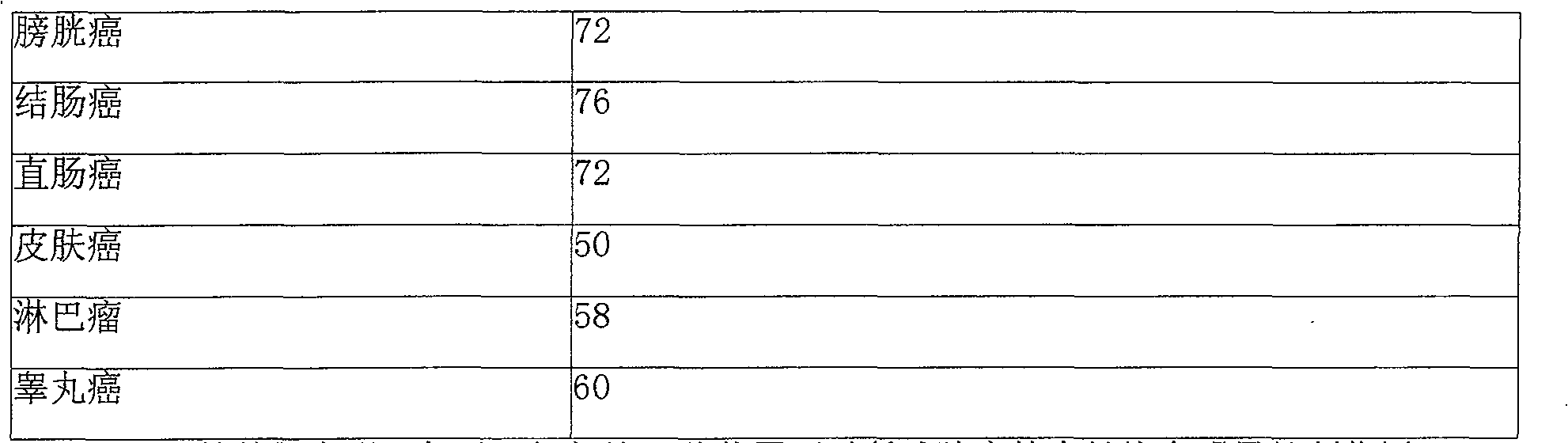
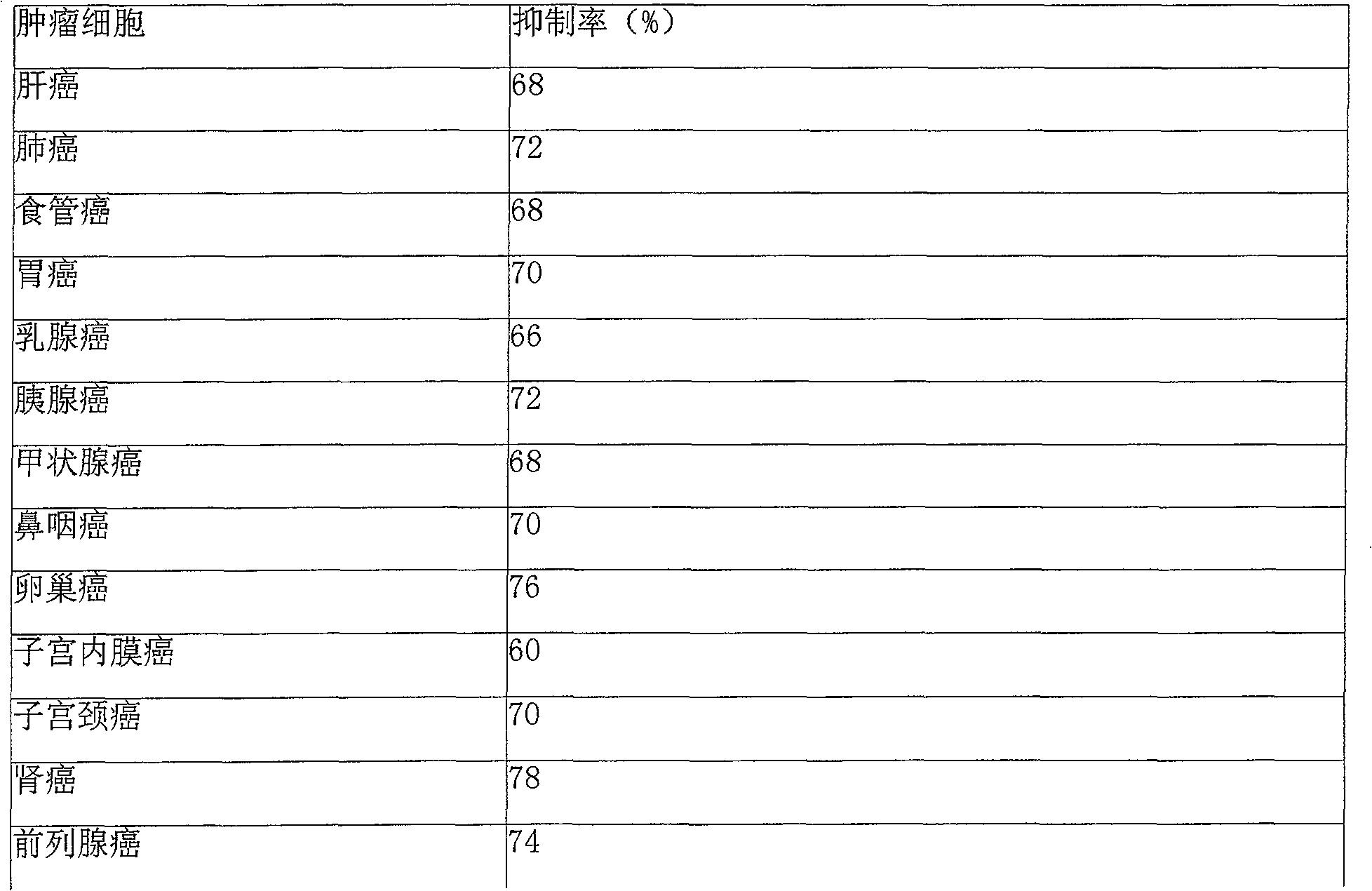Beisalutin sustained-release implantation agent for curing entity tumour
A slow-release implant and tumor technology, applied in the field of medicine, can solve problems such as systemic toxicity and side effects that limit clinical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Put the weighed (60mg) sustained-release excipient (polylactic acid (PLA) with a molecular weight of 10000-20000) into the container, add a certain amount of organic solvent to dissolve and mix (subject to complete dissolution), then add 40mg of Sarotin, re-shake well and vacuum-dry to remove organic solvents. The dried solid composition is shaped immediately, subpackaged and sterilized by radiation to obtain a slow-release implant containing 40% bexarotene. The release time of the sustained-release implant in physiological saline in vitro is 20-26 days, and the release time in mouse subcutaneous is 24-28 days.
Embodiment 2
[0086] Sustained-release implants were made according to the method described in Example 1, but the anti-cancer active ingredients contained were one of the following:
[0087] (A) 1% bexarotene and 99% polylactic acid;
[0088] (B) 5% bexarotene and 95% polylactic acid;
[0089] (C) 10% bexarotene and 90% polylactic acid;
[0090] (D) 15% bexarotene and 85% polylactic acid;
[0091] (E) 20% bexarotene and 80% polylactic acid.
Embodiment 3
[0093]Put the weighed (75mg) sustained-release excipient (PLGA with a molecular weight of 15000-25000, 50:50) into the container, add a certain amount of organic solvent to dissolve and mix (subject to full dissolution), then add 25mg of Besa Rodin, shake again and dry in vacuo to remove organic solvents. The dried solid composition is shaped immediately, subpackaged and sterilized by radiation to obtain a slow-release implant containing 25% bexarotene. The release time of the slow-release implant in physiological saline in vitro is 18-24 days, and the release time in mouse subcutaneous is 16-24 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com