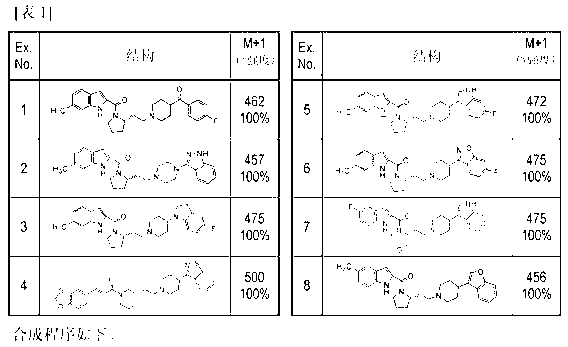
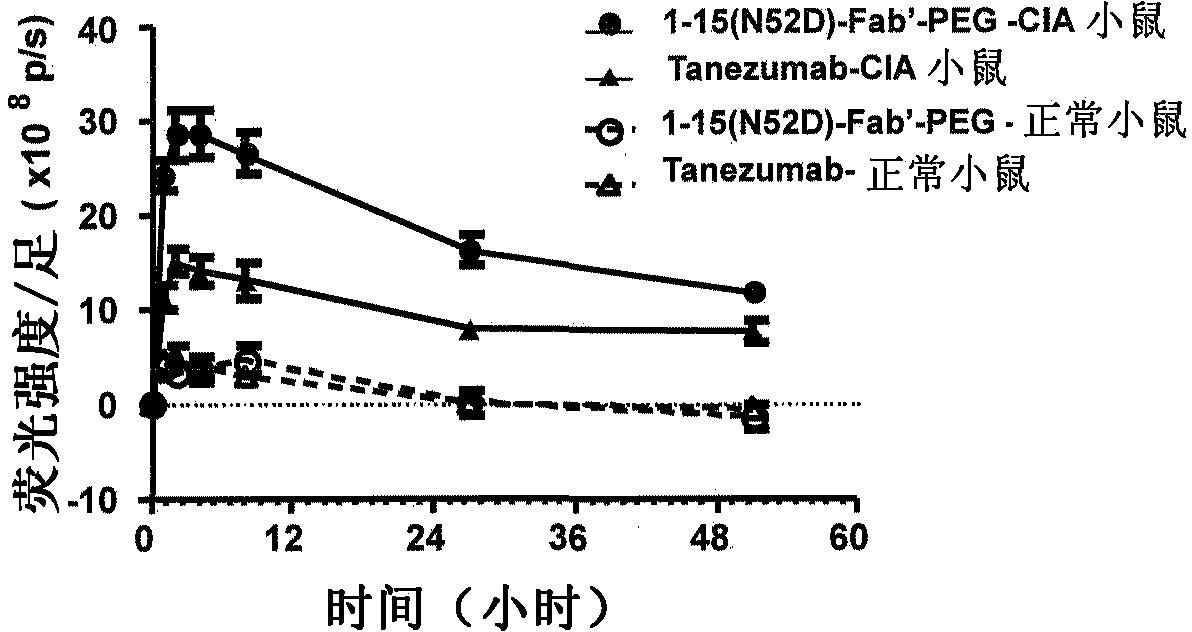
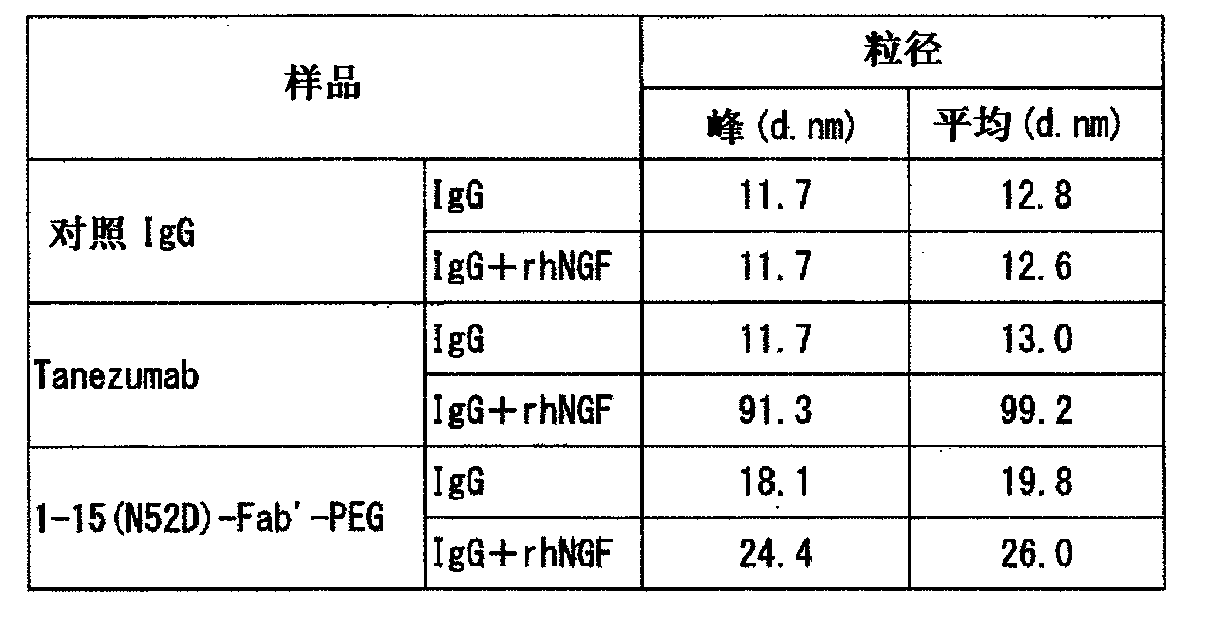
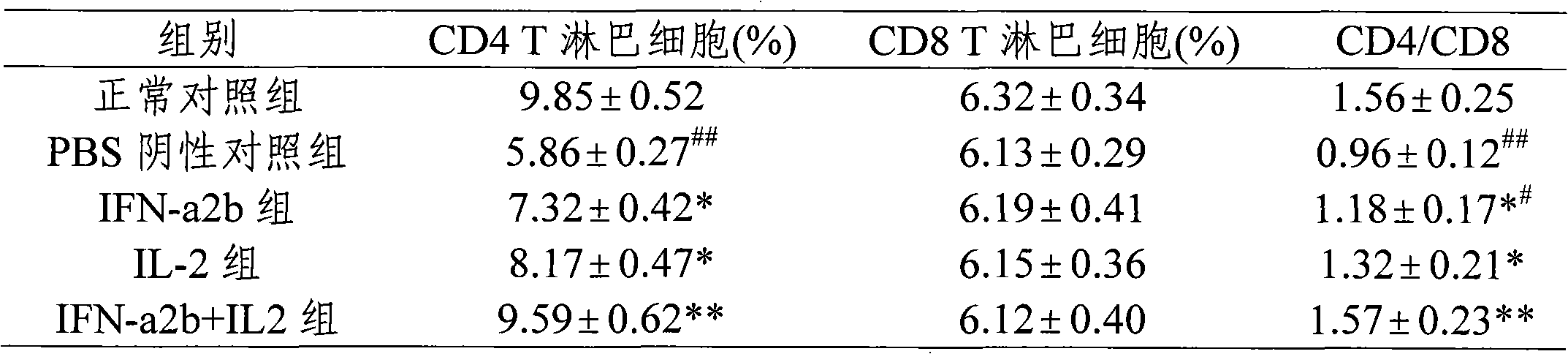
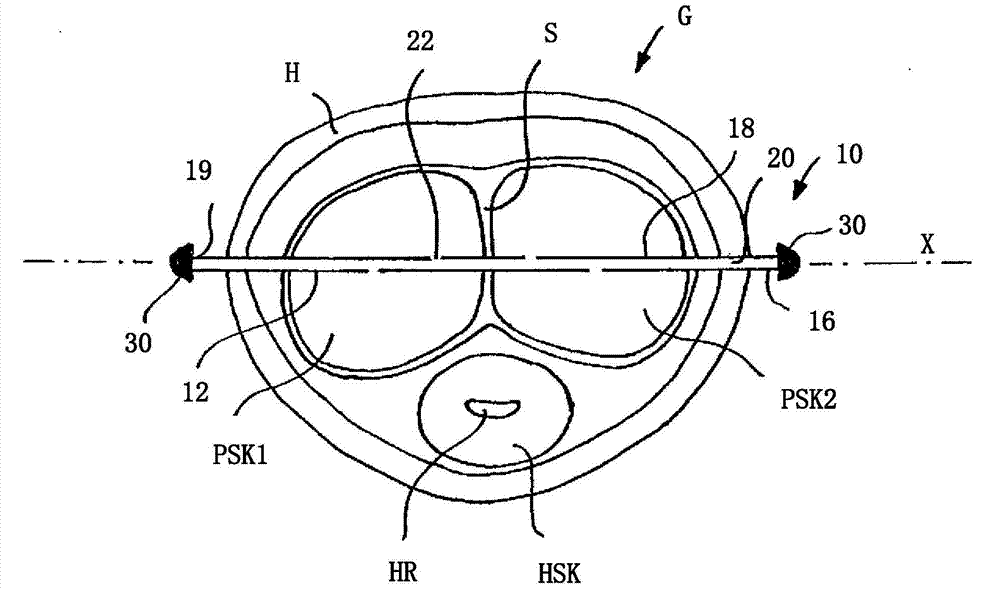
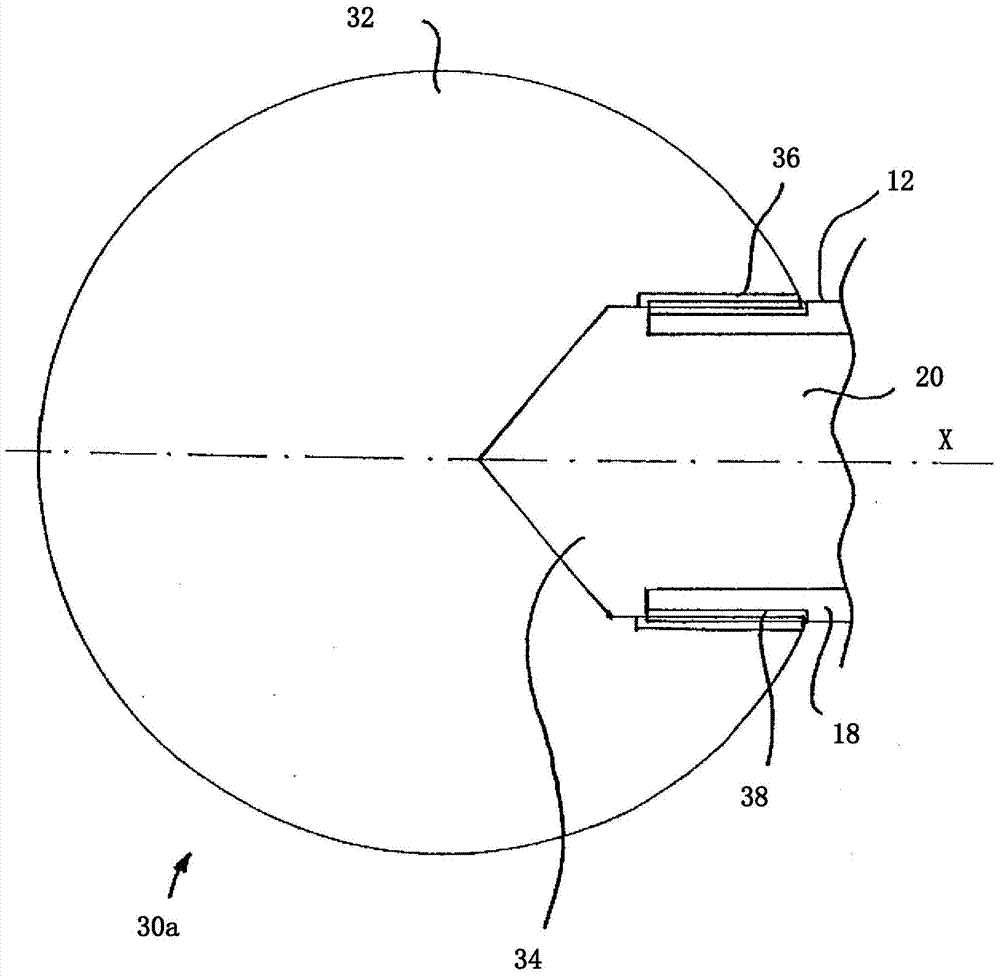
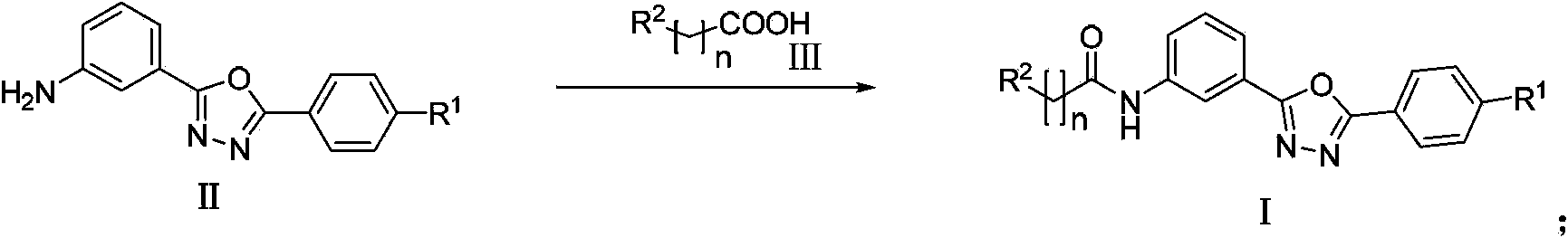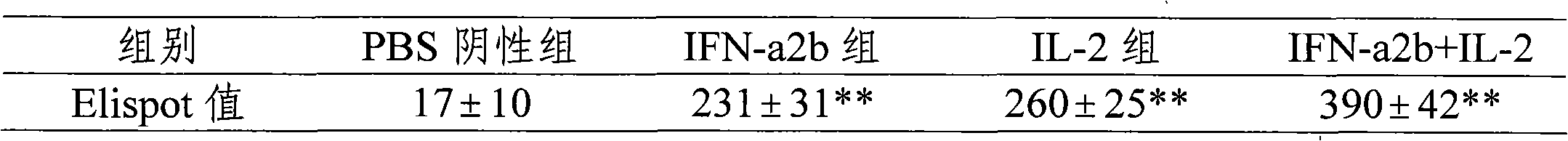Patents
Literature
54results about How to "Reduce the risk of side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
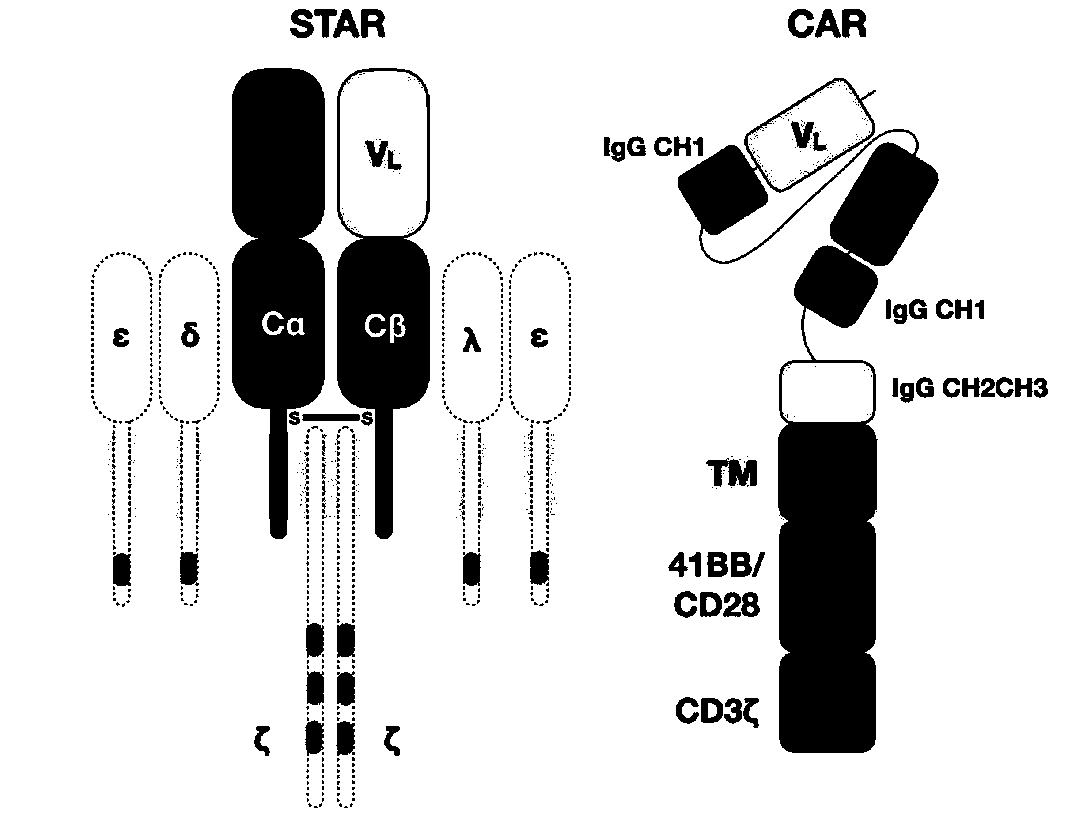
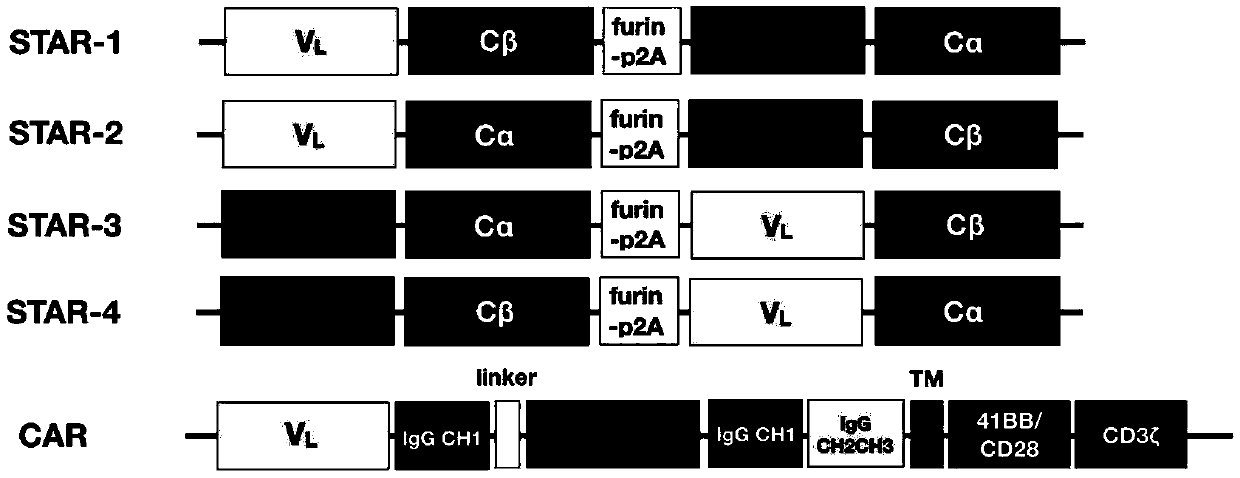
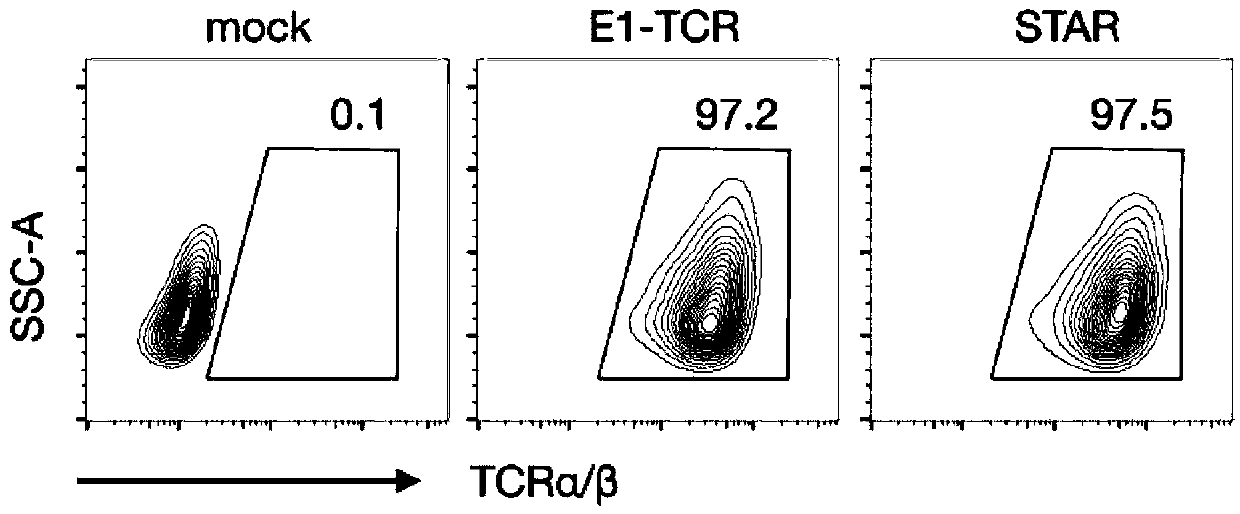
Chimeric T cell receptor STAR and application thereof
ActiveCN110818802AImprove matchReduce the introductionPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsPharmaceutical drug
The invention discloses a chimeric T cell receptor STAR (Synthetic T Cell Receptor and Antigen Receptor), related preparations and drugs, and application of the chimeric T cell receptor STAR in preparation of cell drugs, and also relates to the preparation or an drug composition used for treatment of corresponding diseases such as tumors or infectious diseases.
Owner:CHINA IMMUNOTECH BEIJING BIOTECH CO LTD +2
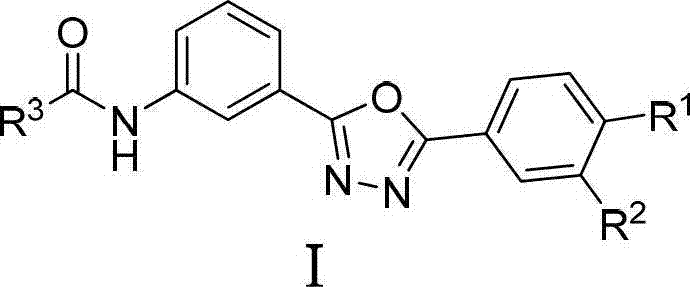
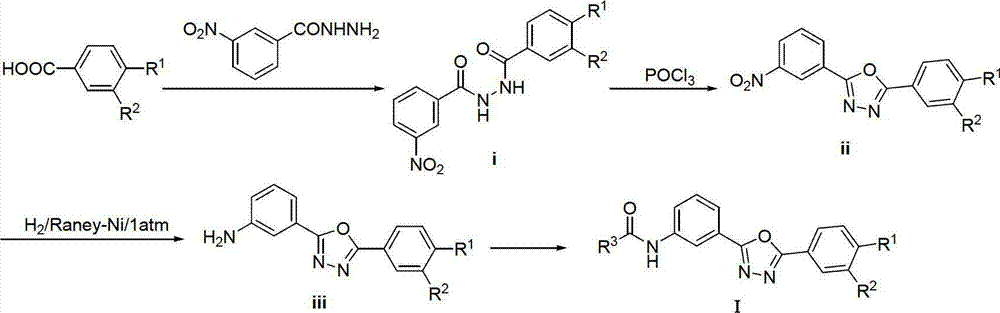
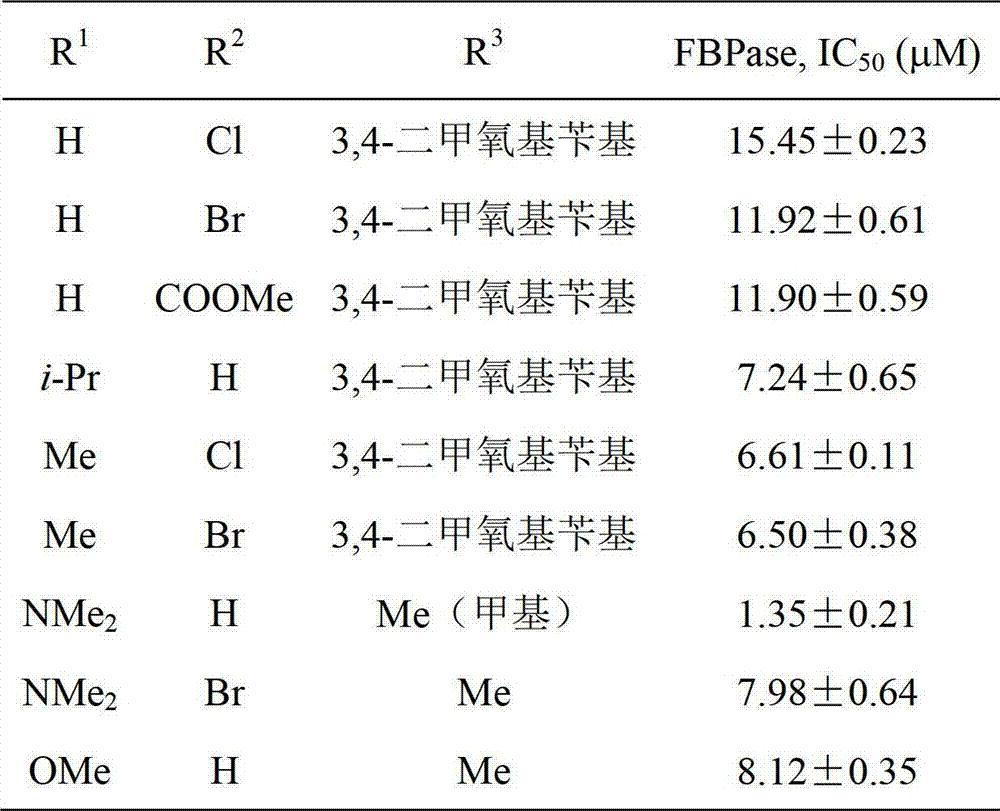
2,5-diaryl-1,3,4-oxadiazole compounds and preparation method and application thereof
InactiveCN102924399AFBPase significantlyReduce the risk of side effectsOrganic active ingredientsOrganic chemistryBenzoic acidAcetic anhydride
The invention relates to 2,5-diaryl-1,3,4-oxadiazole compounds and a preparation method and application thereof. The preparation method comprises the following steps of: condensing benzoic acid of which a 4- site is replaced by R<1> and a 3- site is replaced by R<2> and which is taken as a raw material and m-Nitrobenzoylhydrazine in dichloromethane or tetrahydrofuran to obtain diacylhydrazine i; performing intramolecular dehydration cyclization on the diacylhydrazine i in acetonitrile by taking phosphorus oxychloride as a dehydration reagent to obtain an intermediate ii containing an oxadiazole ring; hydrogenating the intermediate ii under normal pressure under the catalysis of raney nickel to reduce a nitro group to obtain an amino substance iii; and finally, condensing the amino substance iii and acetic anhydride or 3,4-dimethoxyphenylacetic acid under the action of an acid-binding agent to obtain the 2,5-diaryl-1,3,4-oxadiazole compounds I. The invention also discloses application of the 2,5-diaryl-1,3,4-oxadiazole compounds I to the preparation of a medicine for treating type 2 diabetes mellitus.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Environmental pH stimuli-responsive type tumor targeting and controlled drug release nano-carrier and preparation method of nano-carrier
InactiveCN107596385AGood water solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDendrimerNanocarriers
The invention discloses an environmental pH stimuli-responsive type tumor targeting and controlled drug release nano-carrier and a preparation method of the nano-carrier. Dendrimer macromolecular nano-polymer material PAMAM (polyamidoamine) is taken as a main body of a structure and connected with an amphiphilic block copolymer PEG (methoxy polyethylene glycol)-PLA (poly-l-aspartic acid), DOX (doxorubicin) is covalently conjugated to a hydrophobic fragment, namely, PLA, of an arm of the amphiphilic block copolymer and is connected though a pH sensitive hydrazone bond, so that a system has thecharacteristic of in-vivo smart drug release, and drug release can be controlled by in-vivo pH. The connection with a F3 polypeptide nucleolin targeting ligand is realized through a hydrophilic PEG chain segment, and tumor targeting of the system is realized in specific uptake of tumor and nuclear localization. The nano-carrier has potential clinical application value, and powerful technical support is provided for treatment of other types of malignant tumor cells.
Owner:WUHAN UNIV OF TECH
Dural/spinal dural biological patch material, preparation method and application thereof
ActiveCN104130437AWith mechanical propertiesBiocompatibleSurgeryProsthesisPostoperative complicationSide effect
The invention provides a dural / spinal dural biological patch material, a preparation method and application thereof. The obtained biological patch material is especially suitable for repair of dural / spinal dural defect, can maintain stable and non-degradable within 6 months-2 years after being implanted into the human body or animals, and can degrade gradually after more than 2 years. While ensuring certain mechanical properties and liquid seepage resistance, the biological patch material also has biocompatibility, has greatly reduced risks of postoperative complications and side effects, and meets the special requirements for dural / spinal dural defect repair materials.
Owner:YANTAI ZHENGHAI BIO TECH
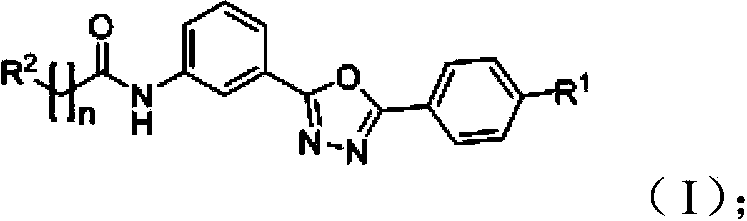
Amidophenyl-1,3,4-oxadiazole compound and its preparation method and use
InactiveCN104098526AGood water solubilityGood membrane permeabilityOrganic active ingredientsOrganic chemistryDiabrezidePhenyl group
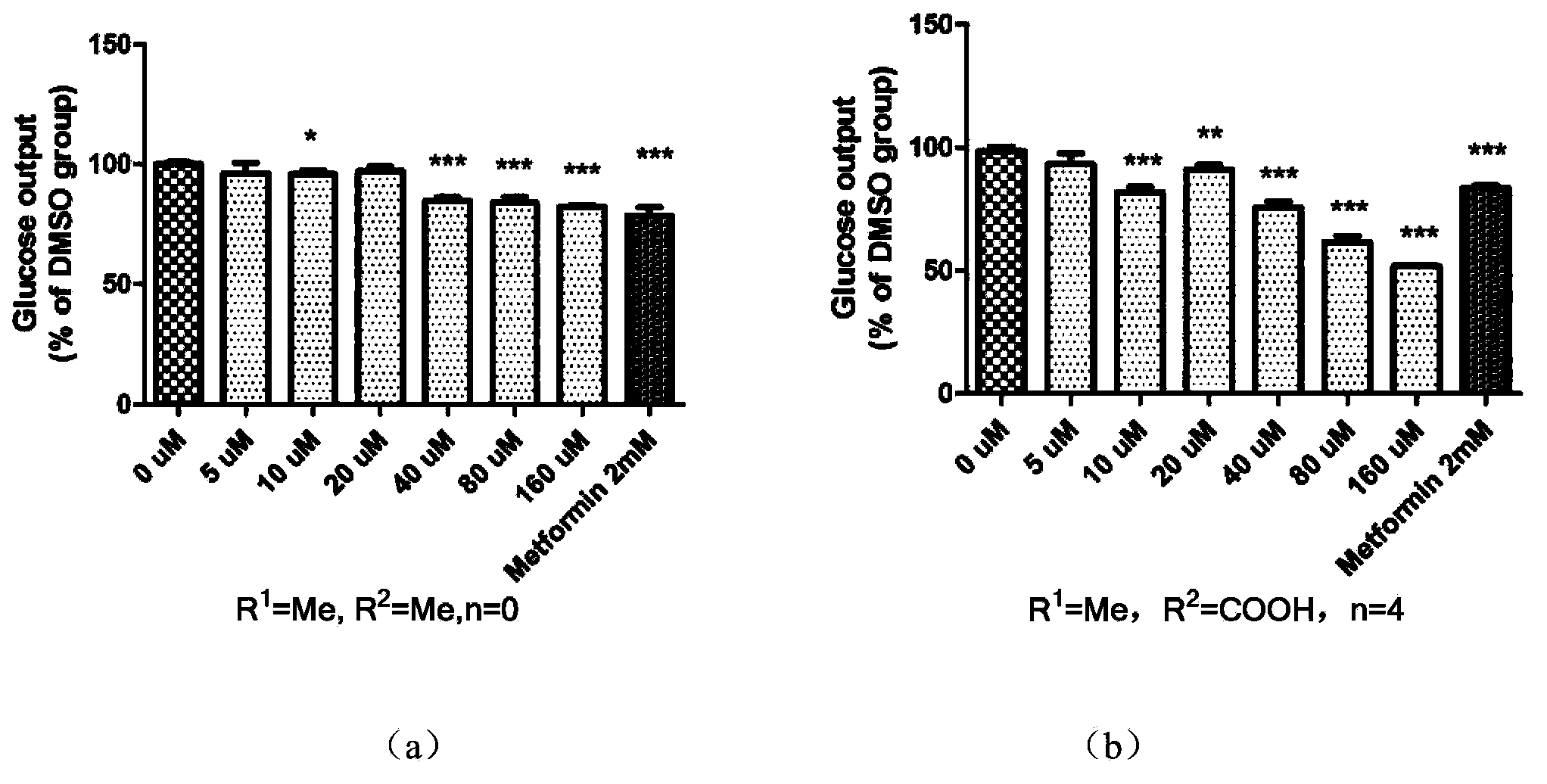
The invention discloses an amidophenyl-1,3,4-oxadiazole compound shown in the formula (I). The invention also discloses a preparation method of the amidophenyl-1,3,4-oxadiazole compound. The preparation method comprises that an amino-compound II and a substituted aliphatic acid III are condensed to form the amidophenyl-1,3,4-oxadiazole compound. The invention also discloses a use of the amidophenyl-1,3,4-oxadiazole compound I in preparation of a drug for treating type II diabetes. The amidophenyl-1,3,4-oxadiazole compound has a non-AMP structure type, can reduce side-effect risk, can obviously inhibit FBPase in a molecule level, can substantially inhibit glucose generation in a cell level, and has good cell viability and good druggability.
Owner:EAST CHINA NORMAL UNIV +1
Astragaloside injection and preparation method thereof
ActiveCN1839920AImprove stabilityQuality is easy to controlOrganic active ingredientsAntiviralsAstragalosideGlycoside formation
The invention relates to an injection using astragalus root glycosides extract as the active ingredient, which also comprises pharmaceutically acceptable adjunct for injection, the content of the astragalus root glycosides in the total astragalus root extract is 80-100%, each unit of the preparation contains astragalus root glycosides 90-300mg.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
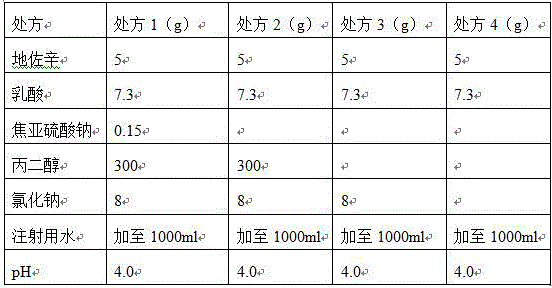
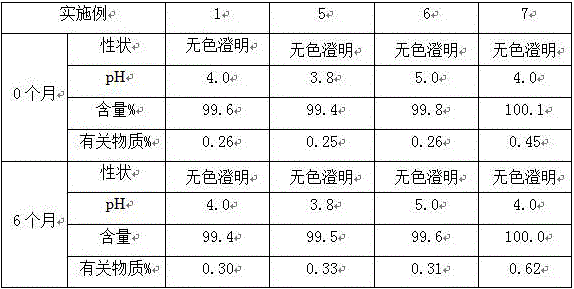
Preparation method of stable dezocine injection
InactiveCN106880586AImprove stabilityReduce the number of typesOrganic active ingredientsNervous disorderSide effectSolvent
The invention provides a preparation method of dezocine injection. The preparation method comprises the following steps: selecting water for injection as the solvent, adding with lactic acid, carrying out stirring for dissolving, and carrying out heating till the temperature is 35-50 DEG C; adding with the medicine, namely, dezocine, and carrying out stirring for clarification; adjusting the pH by adopting sodium hydroxide; and enabling the obtained solution to pass through carbon, carrying out filling, and carrying out high-temperature sterilization, thus obtaining the dezocine injection. For the dezocine injection prepared by adopting the method provided by the invention, the related substance of the medicine is not increased during the preparation process of the injection, meanwhile, three auxiliary materials, namely, sodium pyrosulfite, propylene glycol and sodium chloride are correspondingly reduced, and thus the stability of the injection is further enhanced; moreover, the variety of the used auxiliary materials is reduced, so that the risks generated due to the side effects of clinical medication are reduced, and the preparation method is suitable for the large-scale industrial production.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
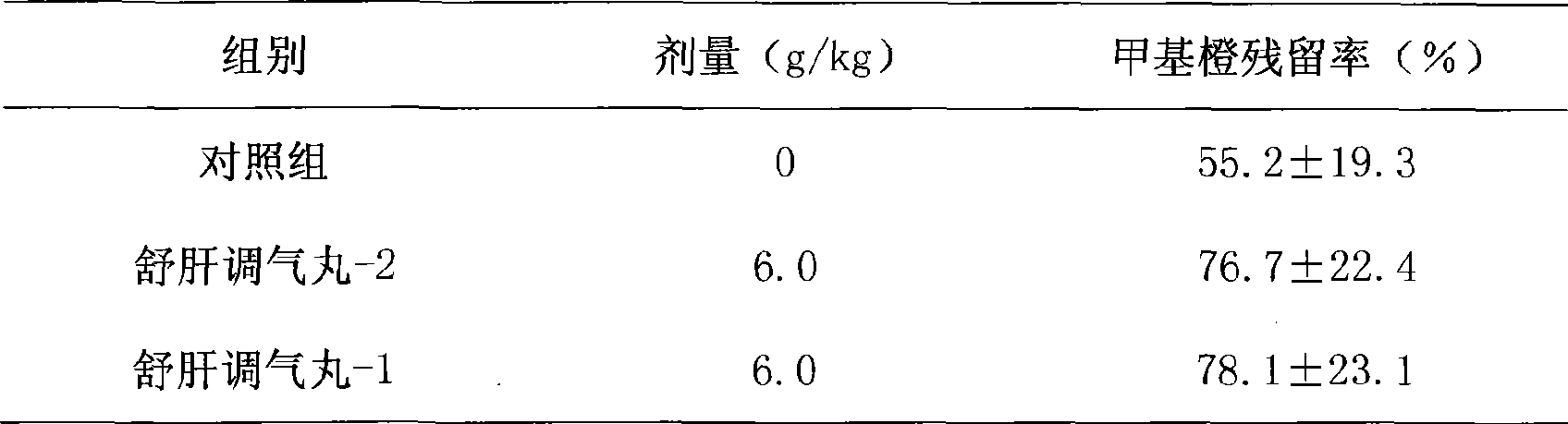
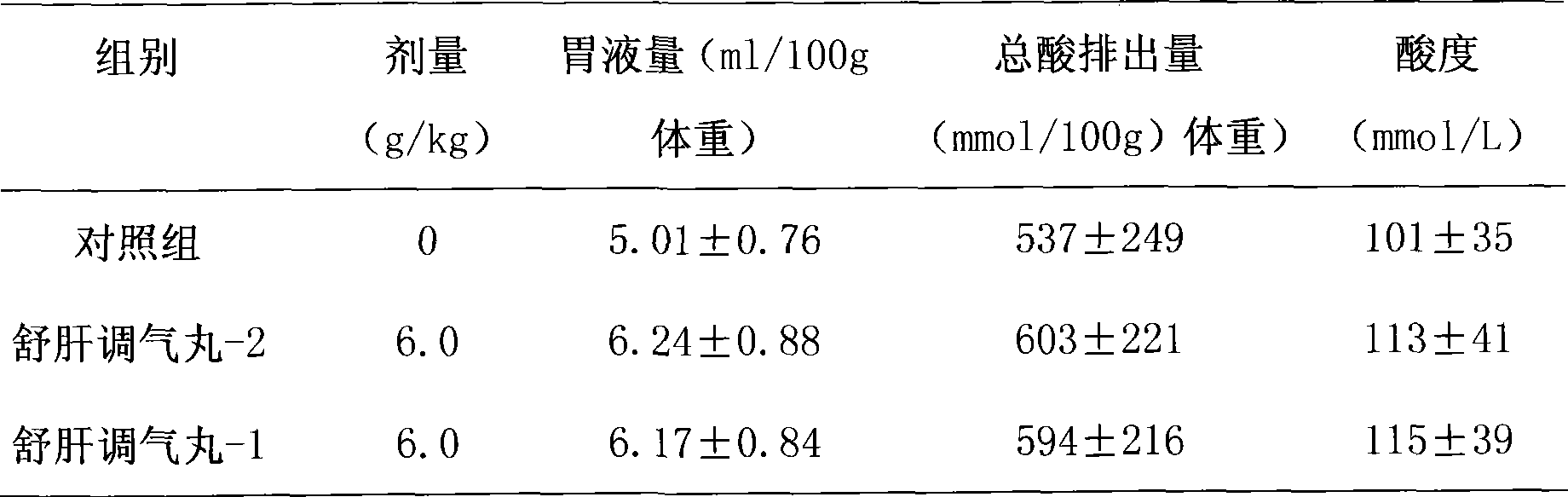
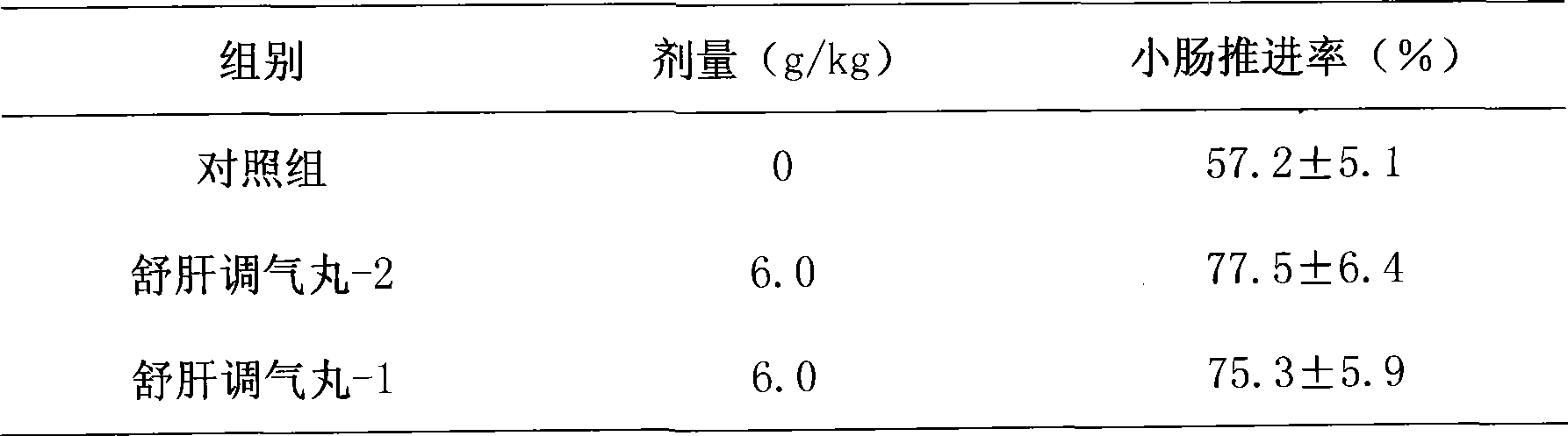
Novel pill for soothing liver and regulating qi
ActiveCN101433694AReduce the risk of side effectsReduce manufacturing costDigestive systemUnknown materialsMedicinal herbsMedicine
The invention discloses a novel liver-soothing Qi-regulating pill, which consists of twenty-one medicinal materials such as Chinese gentian and rhizoma cyperi. The pill is characterized by removing talc on the basis of the prior preparation process and retaining the prior medicinal efficacy. The invention also discloses a preparation method and application of the novel liver-soothing Qi-regulating pill.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
N-acyl cyclic amine derivative or pharmaceutically acceptable salt thereof
InactiveCN102884059AImprove efficiencyReduce the risk of side effectsNervous disorderOrganic chemistryDiseaseSide effect
The present invention provides compounds which show high effectiveness against positive symptoms, negative symptoms and cognitive dysfunction in schizophrenia and reduce conventional side-effect risks as well as have remarkable effects for central neurological diseases associated with cognitive dysfunction other than schizophrenia. N-Acyl cyclic amine derivatives of formula (1): wherein Ar1 and Ar2 are aryl or heteroaryl; V is nitrogen, or CR3; W<1> is a single bond, -C(O)-, etc.; W<2> is C1- alkylene; W3 is a single bond, methylene, -NH-, -CR<4>=CR<5>-, etc.; Ring Q is a group of formula (a) in which n is 0 or 1; m is 0 to 2; k is 1 to 3; Z is a single bond, methylene, oxygen, etc.; R<1a>, R<1b> and R<1c> are each, same or different, hydrogen, hydroxyl, halogen, cyano, C1-6 alkyl, etc.
Owner:SUMITOMO PHARMA CO LTD
Novel anti-human NGF antibody
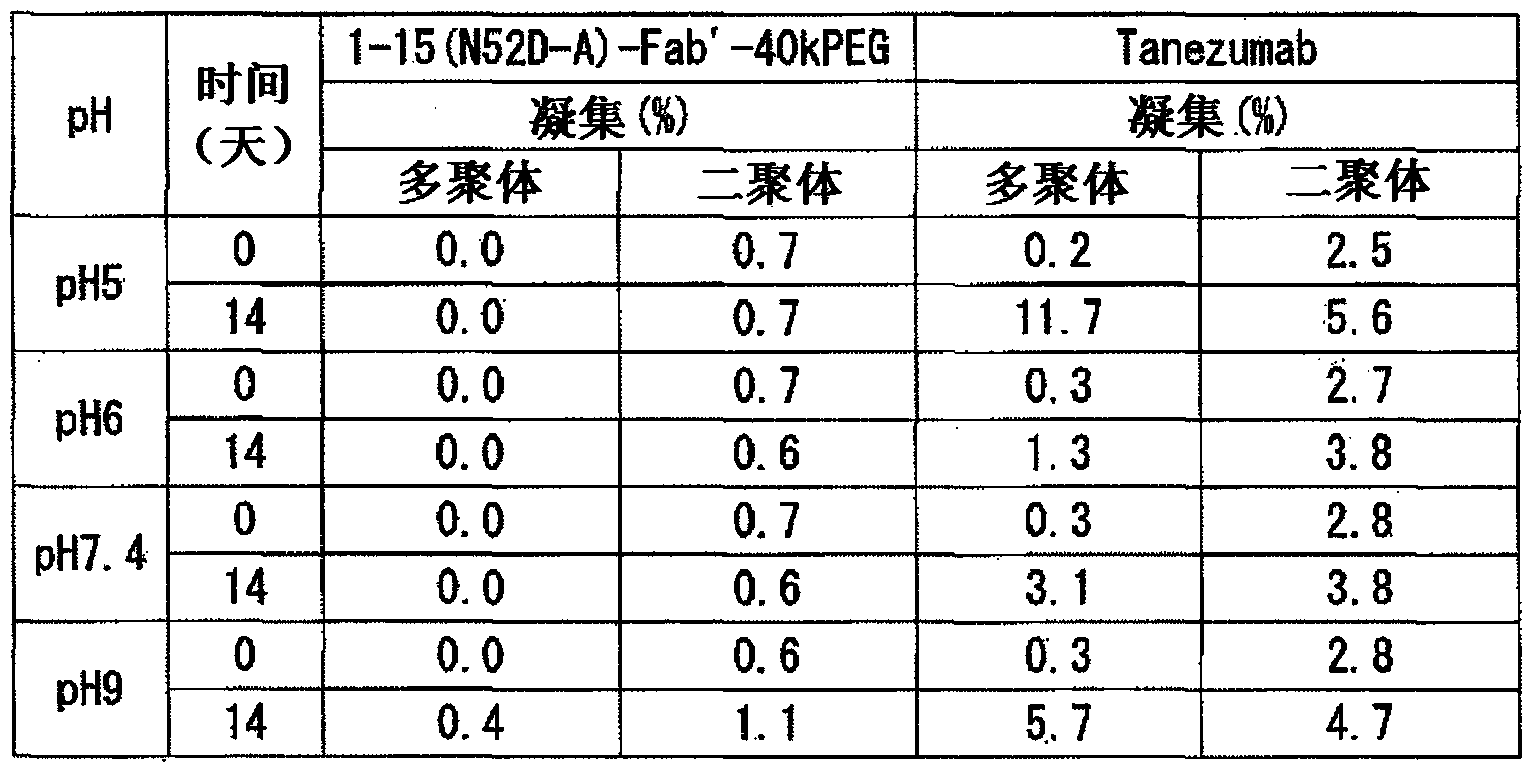
Provided are an anti-human NGF antibody which is reduced in the influence on fetuses and the risk of adverse side effects including thrombosis while keeping a high neutralizing activity, and which has excellent safety, or an antigen-binding fragment thereof; and a means, utilizing the antibody or an antigen-binding fragment thereof , for preventing or treating various diseases for which human NGF is involved in the development of a disease state. An Fab' fragment of an anti-human NGF antibody comprises a heavy-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 6 and a light-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 4.
Owner:ASTELLAS PHARMA INC
Traditional Chinese medicine composition for relaxing bowels, preparation method and application thereof
PendingCN109939168ASimple ingredientsAvoid overreactionDigestive systemPlant ingredientsSide effectAngelica Sinensis Root
The invention discloses a traditional Chinese medicine composition for relaxing bowels and a preparation method and application thereof. The active ingredients of the traditional Chinese medicine composition are prepared from the extracts of medicinal raw materials, and the medicinal raw materials include aloe and angelica sinensis with the mass ratio of (2-5) to (1.5-12). By applying the technical scheme of the invention, the traditional Chinese medicine composition for relaxing bowels is simple in composition, the effective ingredients are extracted from the aloe and angelica sinensis, possibly existing excessive reactions of complicated ingredients are avoided, the risk of side effects generated after medicine taking is obviously reduced, and the traditional Chinese medicine compositionhas a good bowel relaxing effect.
Owner:TSING HUA DE REN XIAN HAPPINESS PHARMA
Medicinal composition for treating diseases caused by enterovirus infections
ActiveCN102309758AReduce dosageReduce the risk of side effectsPeptide/protein ingredientsDigestive systemHand-foot-and-mouth diseaseAntiviral drug
The invention relates to a medicinal composition, which particularly consists of a first agent of an antiviral medicament and a second agent of an immunomodulator, wherein the first agent, which is prepared by blocking virus messenger ribose nucleic acid (mRNA) translation and inhibiting the synthesis of virus nucleic acid in a cell. The medicinal composition can be used for treating organic pathological changes caused by enterovirus infections, such as a hand, foot and mouth disease.
Owner:BEIJING KAWIN TECH SHARE HLDG
Piercing-like refillable drug feeding device
The invention relates to a piercing-like device (10) for feeding a drug, for example into the penis for the purpose of treating erectile dysfunctions, comprising an elongate hollow body (12) having a first longitudinal end (14) and a second longitudinal end (16), wherein the hollow body (12) comprises a radially outer peripheral wall (18), which surrounds a radially inner, elongate hollow body (20). The peripheral wall (18) of the hollow body (12) has at least one drug outlet opening (22) between the two longitudinal ends (14, 16).
Owner:A·施密特
Surface coated positive electrode material and preparation method thereof, and battery
ActiveCN109755551AReduce direct contact areaReduce riskCell electrodesSecondary cellsPhysical chemistrySide reaction
The invention relates to a surface coated positive electrode material and a preparation method thereof, and a battery; the preparation method comprises the following steps of surface treatment, metalion adsorption, deposition treatment and oxidation sintering. The method has the beneficial effects that the self-adsorption of metal cations is utilized, so that the obtained coating layer is uniformand complete, the direct contact area of the active substance and the electrolyte can be greatly reduced, and the risk of side reaction is reduced; the in-situ growth mechanism is adopted, so that the size of the deposited particles can be regulated and controlled, the size is between the nanometer crystal and micron interval, and the compactness of the coating layer is controlled; the thicknessof the coating layer can be adjusted and controlled by adjusting the number of deposition times of in-situ growth, so that a better protection effect can be obtained; an agglomeration phenomenon occurring during conventional wet-process coating can be avoided; an and the in-situ coating method is adopted, and severe stirring is not needed, so that physical crushing caused by secondary ball formingis avoided.
Owner:湖南桑瑞新材料有限公司
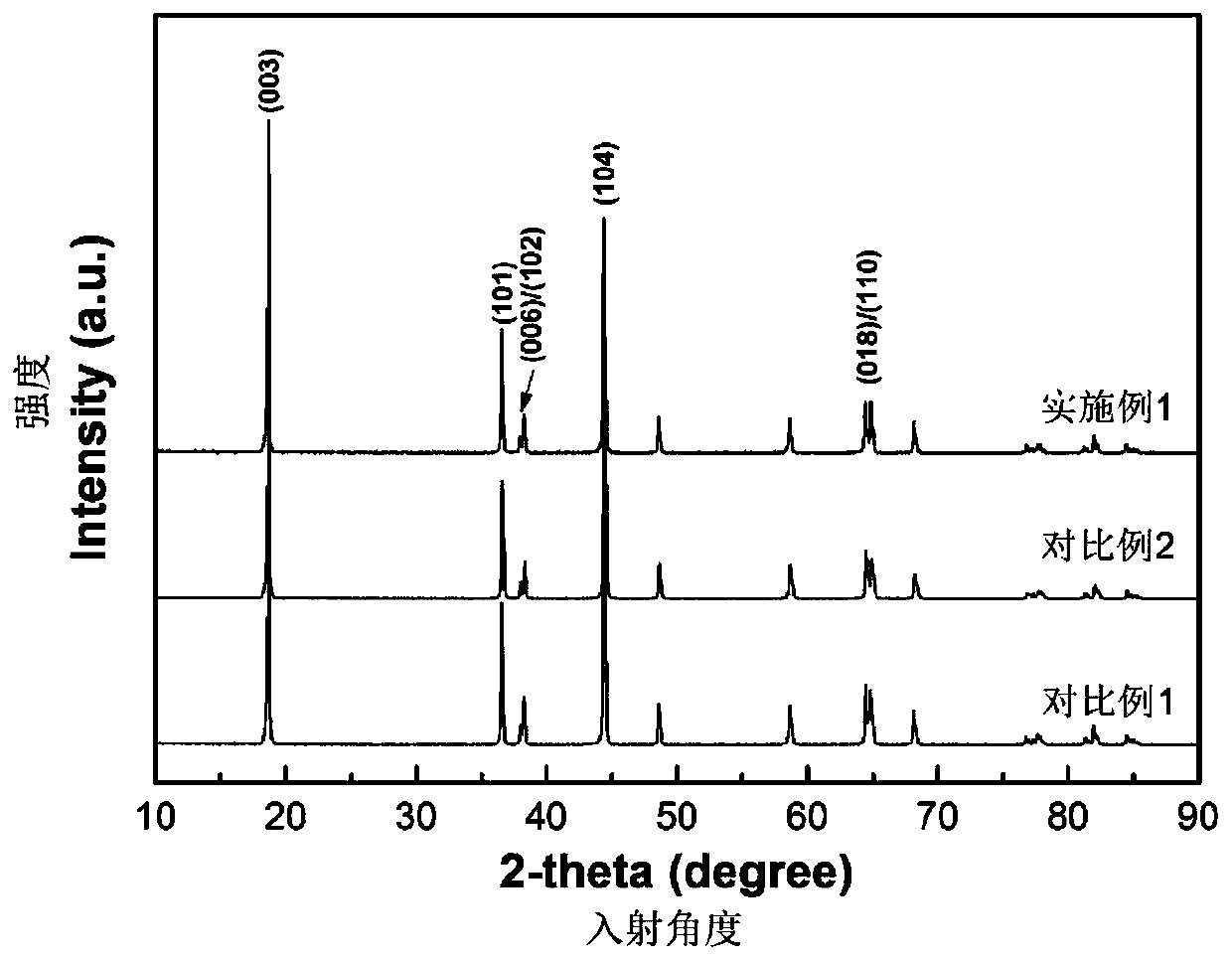
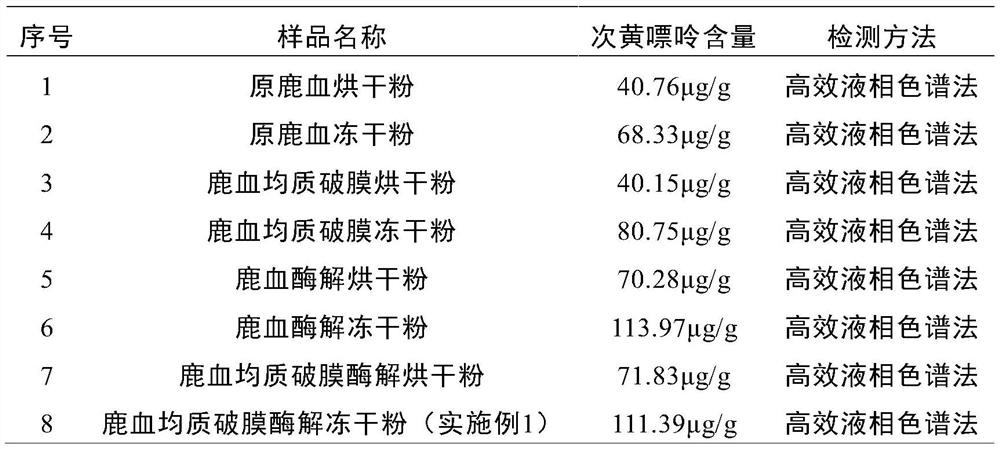
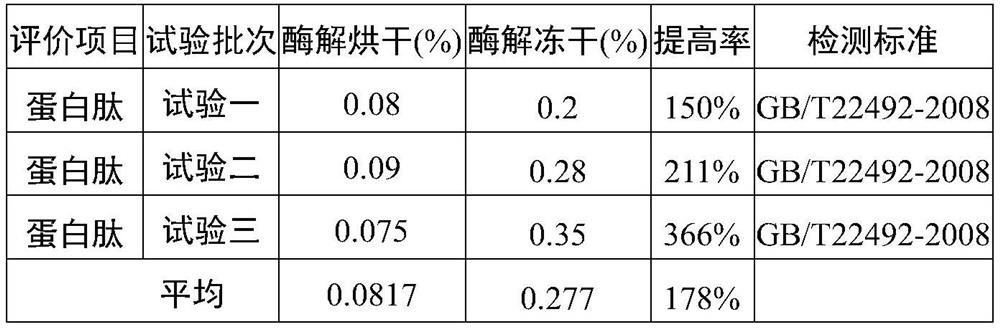
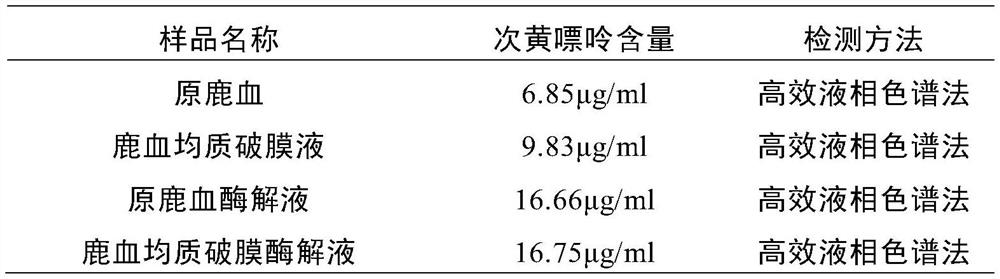
Deer blood product and preparation method thereof
PendingCN112791103AImprove performanceHigh purityPowder deliveryNervous disorderBiotechnologyAnimal science
The invention relates to a deer blood product and a preparation method thereof. The preparation method of the deer blood product provided by the invention comprises the following steps: performing membrane rupture and enzymolysis on deer blood to obtain deer blood membrane rupture enzymatic hydrolysate; and carrying out freeze drying on a raw material containing the deer blood membrane rupture enzymatic hydrolysate by taking lactose and / or trehalose as a freeze-drying protective agent, wherein the weight ratio of the deer blood to the freeze-drying protective agent is (70-80): (8-15). The deer blood product is prepared from the following raw materials: fresh deer blood, lactose, a nutritional supplement, trehalose and a preservative. According to the deer blood product prepared by the invention, the active effective components are greatly increased, the preparation process is advanced, the product purity is high, impurities are few, no chemical preservative is added, the taste is improved, the application effect, quality, safety and reliability of the deer blood product are improved, and industrial production and popularization are facilitated.
Owner:SIPING HUAKE BIOLOGICAL TECH
Pharmaceutical composition with synergistic anti-melanoma effect and application thereof
ActiveCN110664807AEffective against melanomaReduce dosageAldehyde active ingredientsAntineoplastic agentsSide effectEfficacy
The invention discloses a pharmaceutical composition with a synergistic anti-melanoma effect and an application of the pharmaceutical composition, and the pharmaceutical composition comprises cinnamylaldehyde and dacarbazin. The composition disclosed by the invention has a relatively good melanoma resisting effect; the pathological experiments combined with the use of related mouse transplanted tumor models in the research of tumor pathogenesis find that cinnamaldehyde and dacarbazin are combined to achieve a strong effect of inhibiting the growth of solid tumors, and cinnamaldehyde and dacarbazin have a synergistic effect, so that the dosage of dacarbazin can be reduced, the risk of side effects caused by chemotherapeutics is reduced, and the pharmaceutical composition is applied to treatment of melanoma.
Owner:NANKAI UNIV
Traditional Chinese medicine composition for relaxing bowels, preparation method and application thereof
PendingCN109939167ASimple ingredientsAvoid overreactionDigestive systemPlant ingredientsSide effectAdditive ingredient
The invention discloses a traditional Chinese medicine composition for relaxing bowels and a preparation method and application thereof. The active ingredients of the traditional Chinese medicine composition are prepared from the extracts of medicinal raw materials, and the medicinal raw materials include aloe and angelica sinensis according to the mass ratio of (2-5) to (1-10). By applying the technical scheme of the invention, the traditional Chinese medicine composition for relaxing bowels is simple in composition, the effective ingredients are extracted from the aloe and fructus aurantii,possibly existing excessive reactions of the complicated ingredients are avoided, the risk of side effects generated after medicine taking is obviously reduced, and the traditional Chinese medicine composition has a good bowel relaxing effect.
Owner:TSING HUA DE REN XIAN HAPPINESS PHARMA
Cd44 binding peptides
InactiveCN106061496AReduce the risk of side effectsPowder deliveryNervous disorderNanoparticleBinding peptide
The present invention relates to a protein which binds to the domain encoded by exon 9 of human CD44 (CD44ex9), to fusion proteins and conjugates of said protein and especially to nanoparticles conjugated to said protein. The invention further relates to a method of production for the protein and the respective conjugated nanoparticles and the use of the protein of the invention for treatment and diagnosis of cancer diseases.
Owner:EXCHANGE IMAGING TECH GMBH
Preparation method of precursor of ibrutinib
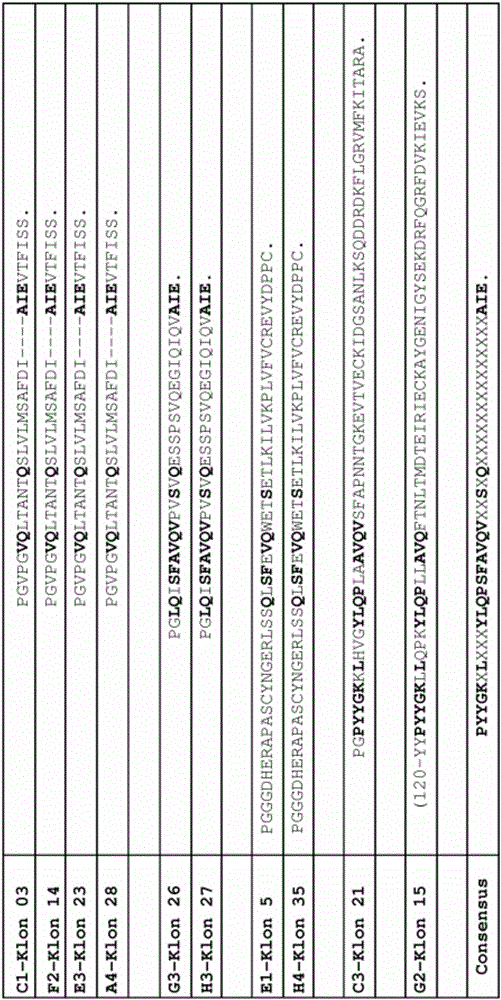
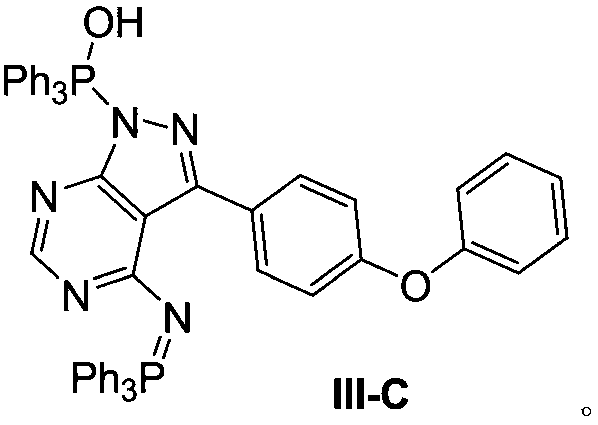
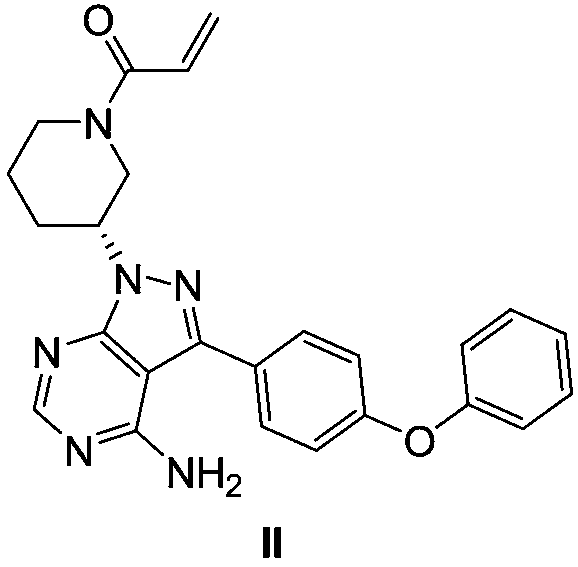
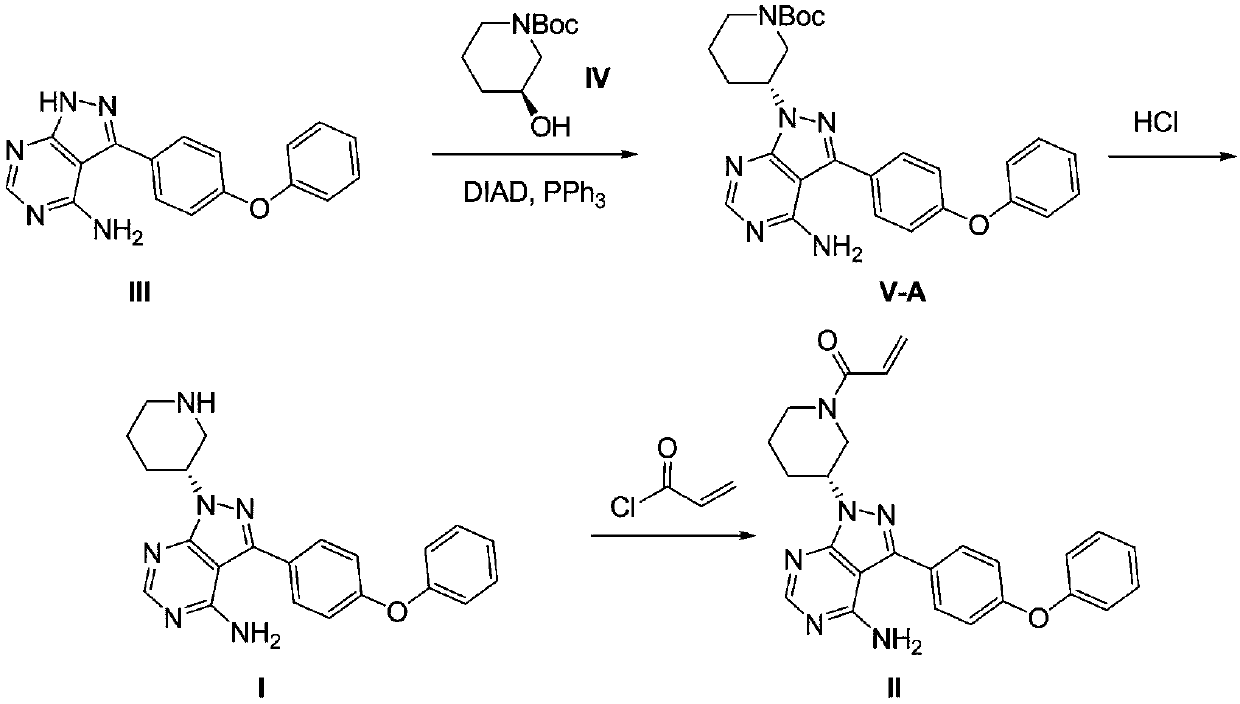
ActiveCN111004239AEasy to manufactureReduce the risk of side effectsGroup 5/15 element organic compoundsPtru catalystPharmaceutical industry
The invention relates to the pharmaceutical industry, in particular to a preparation method of a drug intermediate, and specifically discloses a preparation method of a precursor of ibrutinib. The preparation method comprises the following steps: (1) reacting a compound (III) with triphenylphosphine and azodicarbonic acid diester to obtain an intermediate (III-B); (2) reacting the intermediate (III-B) with a compound (IV) under the action of a catalyst to obtain an intermediate (V-C); and (3) reacting the intermediate (V-C) under the action of hydrochloric acid to obtain (R)-3-(4-phenoxy phenyl)-1-(piperidine-3-yl)-1H-pyrazolo [3, 4-d] pyrimidine-4-amine (I). The method has the advantages of high yield, high purity, convenience in purification, simplicity and convenience in operation and the like, is suitable for industrial production, and contributes to reducing the cost to a certain extent.
Owner:上海柏狮生物科技有限公司
Artemisia apiacea essential oil pet deodorization antibacterial liquid and preparation method thereof
PendingCN112870241AEliminate side effectsDelay drug resistanceAntibacterial agentsCosmetic preparationsBiotechnologyAnti bacterial
The invention provides an artemisia apiacea essential oil pet deodorization antibacterial liquid and a preparation method thereof. The artemisia apiacea essential oil pet deodorization antibacterial liquid comprises the following components in percentage by weight: 5-15% of artemisia apiacea essential oil, 5-10% of a deodorant, 3-8% of an antibacterial agent and the balance of water, the method comprises the following steps: firstly, collecting an artemisia apiacea stock solution, taking artemisia apiacea leaves, cutting the artemisia apiacea leaves into small pieces, putting the small pieces into a leaf mashing chamber, fully mashing, adding ethanol, stirring, extruding and filtering to obtain the artemisia apiacea stock solution, and flowing into a refining chamber; refining the artemisia apiacea stock solution: adding water into the artemisia apiacea stock solution in the refining chamber, heating and stirring, precipitating for 5-10 minutes after stirring completely, and obtaining supernatant through a suction guide pipe to obtain artemisia apiacea essential oil; and adding a deodorant, an antibacterial agent and water into the prepared artemisia apiacea essential oil in proportion, and fully stirring to obtain the pet deodorization antibacterial liquid containing the artemisia apiacea essential oil.
Owner:HENAN HEIMA ANIMAL PHARM CO LTD
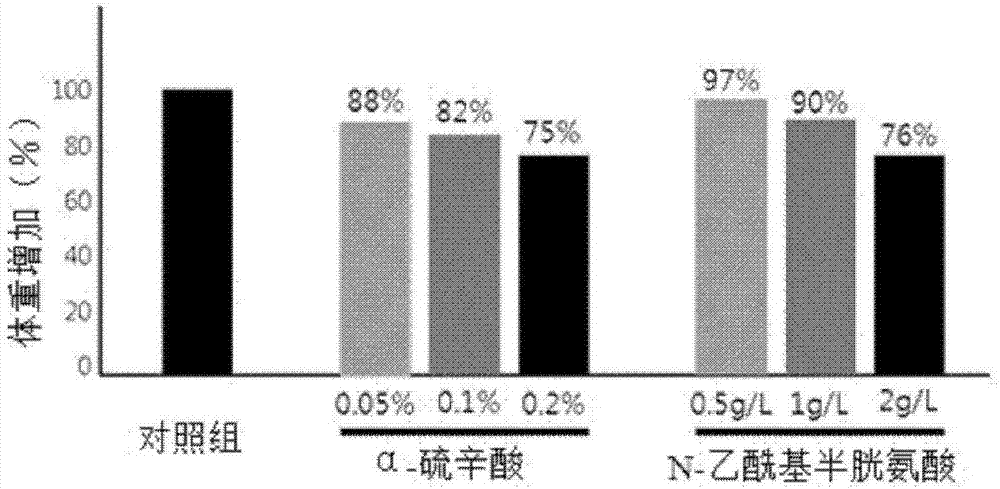
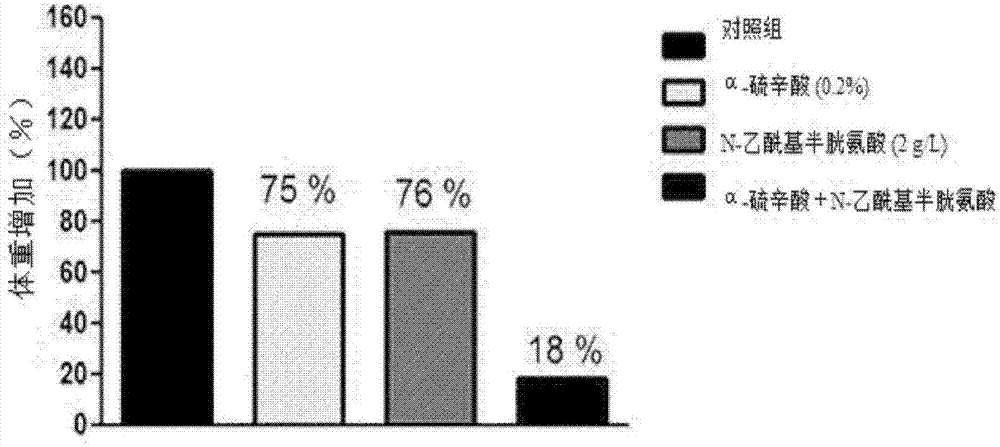
Composition for preventing or treating obesity containing alpha-lipoic acid and n-acetylcysteine as active ingredients
InactiveCN107106540AReduce the risk of side effectsIncrease weightPowder deliveryOrganic active ingredientsWeight gainingSide effect
Owner:THE ASAN FOUND
Polypeptide for preventing novel coronavirus pneumonia COVID-19, immunogenic conjugate and application thereof
PendingCN114057850ALow site requirementsSmall and simple molecular structureSsRNA viruses positive-senseAntibody mimetics/scaffoldsAdjuvantVaccine Production
The invention relates to the fields of immunological technology, biotechnology and biological medicine, in particular to a polypeptide for preventing novel coronavirus pneumonia COVID-19, an immunogenic conjugate and application of the polypeptide and the immunogenic conjugate. The immunogenic conjugate comprises a polypeptide with an amino acid sequence as shown in SEQ ID NO.1, and is used for preparing a vaccine for preventing COVID-19. The immunogenic conjugate is a synthetic peptide vaccine prepared by synthesizing a target polypeptide based on a known or predicted section of antigen epitope amino acid sequence in a pathogen antigen gene, combining the synthesized target polypeptide with a carrier protein to prepare a polypeptide conjugate, and adding an adjuvant. Compared with a traditional attenuated vaccine, an inactivated vaccine and other novel vaccines, the vaccine can quickly cope with virus variation; the field requirement for vaccine production is reduced, and large-scale batch production is easy to realize; the research and development cost and the research and development period are reduced; safety, no toxicity and stability are realized; as the molecular structure is small and simple, the problems of serious complications and iatrogenic infection are rarely caused.
Owner:TSINGHUA UNIV +1
Low-immunogenicity anti-TNF-alpha humanized monoclonal antibody TCX063 and application thereof
ActiveCN111909267ALower requirementAvoid clearingAntipyreticAnalgesicsPharmaceutical drugImmunogenicity
The invention relates to the technical field of biology, in particular to a low-immunogenicity anti-TNF-alpha humanized monoclonal antibody TCX063 and an application thereof. The anti-TNF-alpha humanized monoclonal antibody TCX063 having reduced immunogenicity, provided by the invention is obtained by modifying an FR region of an Infliximab monoclonal antibody. The antibody provided by the invention has the same TNF-alpha antigen binding site as that of the Infliximab monoclonal antibody, and retains the antigen affinity and specificity of the Infliximab monoclonal antibody, but the immunogenicity is obviously lower than that of the Infliximab monoclonal antibody. The low-immunogenicity TNF-alpha antibody can reduce the risk of drug side effects caused by immune response caused by antibodyimmunogenicity original nature in a human body. The antibody drug ADA is reduced. The in-vivo half-life period of the antibody is prolonged. Besides, a subcutaneous administration mode becomes possible, and great application potential and value are achieved.
Owner:ABMAX BIOTECHNOLOGY CO LTD
Surgical protective device
PendingCN114305715AReduce the risk of side effectsReduce the probability of exposureOperating tablesSurgical drapesReoperative surgeryMedical treatment
The invention belongs to the technical field of medical instruments, and particularly relates to a surgical protective device which comprises an upper-layer pad, an upper-layer opening supporting ring, an upper-layer connecting strip, a lower-layer pad, a lower-layer opening supporting ring and a lower-layer connecting seat. Openings are formed in the upper-layer pad and the lower-layer pad, the positions of the openings of the upper-layer pad and the lower-layer pad are opposite, the upper-layer opening supporting ring is detachably connected to the opening of the upper-layer pad, the upper end of the upper-layer connecting strip is installed on the upper-layer opening supporting ring, and the lower-layer opening supporting ring is detachably connected to the opening of the lower-layer pad. The lower end of the lower layer connecting base is fixedly installed on the lower layer opening supporting ring and detachably connected with the lower end of the upper layer connecting strip, and at least two ropes are further arranged at the lower end of the lower layer opening supporting ring. The lower-layer opening supporting ring and the upper-layer opening supporting ring are positioned through the upper-layer connecting strip, the lower-layer connecting base and the rope, so that the protection effect is achieved, and the trouble that a protection pad needs to be fixed manually in the whole operation process is reduced.
Owner:李奇
Polypeptide for preventing novel coronavirus pneumonia COVID-19, immunogenic conjugate and application thereof
PendingCN114057843ALow site requirementsSmall and simple molecular structureSsRNA viruses positive-senseViral antigen ingredientsAdjuvantVaccine Production
The invention relates to the fields of immunological technology, biotechnology and biological medicine, in particular to a polypeptide for preventing novel coronavirus pneumonia COVID-19, an immunogenic conjugate and application of the polypeptide and the immunogenic conjugate. The immunogenic conjugate comprises a polypeptide with an amino acid sequence as shown in SEQ ID NO.1, and is used for preparing a vaccine for preventing COVID-19. The immunogenic conjugate is a synthetic peptide vaccine prepared by synthesizing a target polypeptide based on a known or predicted section of antigen epitope amino acid sequence in a pathogen antigen gene, combining the synthesized target polypeptide with a carrier protein to prepare a polypeptide conjugate, and adding an adjuvant. Compared with a traditional attenuated vaccine, an inactivated vaccine and other novel vaccines, the vaccine can quickly cope with virus variation; the field requirement for vaccine production is reduced, and large-scale batch production is easy to realize; the research and development cost and the research and development period are reduced; safety, no toxicity and stability are realized; as the molecular structure is small and simple, the problems of serious complications and iatrogenic infection are rarely caused.
Owner:TSINGHUA UNIV +1
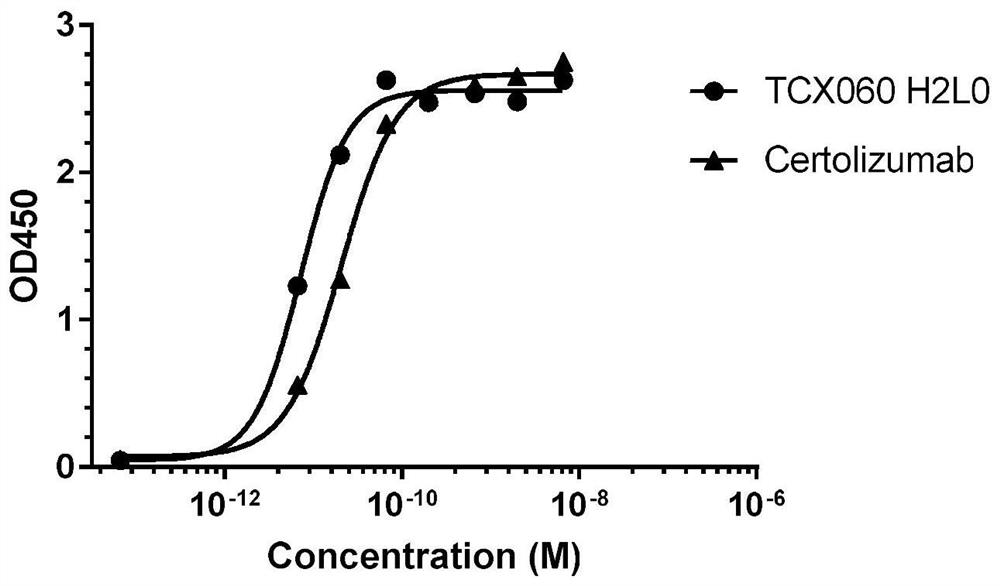
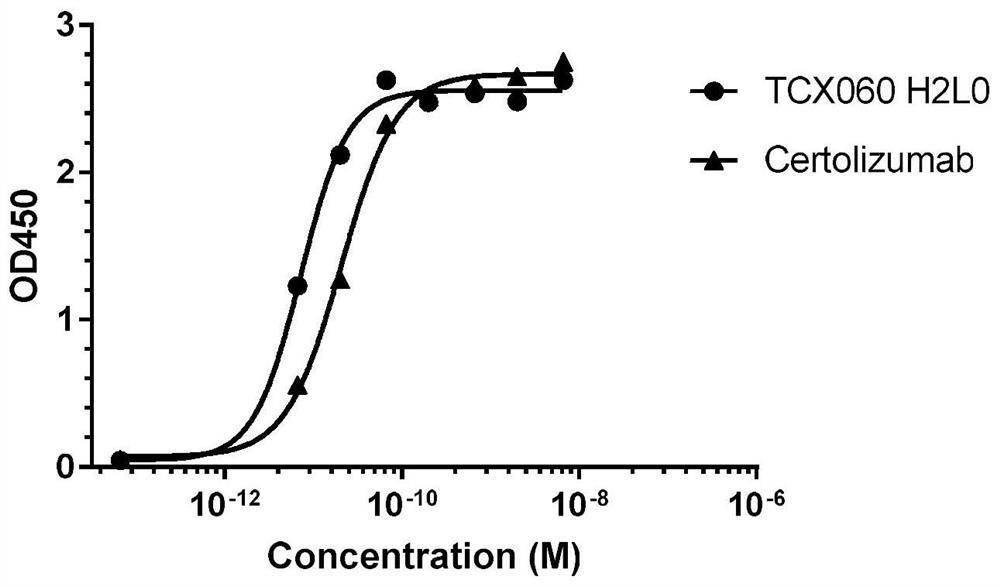
Anti-TNF-alpha humanized monoclonal antibody TCX060 having low immunogenicity and low ADCC/CDC function and application of anti-TNF-alpha humanized monoclonal antibody TCX060
ActiveCN111909268ASimple production processReduce the risk of side effectsAntipyreticAnalgesicsAntiendomysial antibodiesImmunogenicity
The invention relates to the technical field of biology, in particular to an anti-TNF-alpha humanized monoclonal antibody TCX060 having low immunogenicity and low ADCC / CDC function and an applicationof the anti-TNF-alpha humanized monoclonal antibody TCX060. The anti-TNF-alpha humanized monoclonal antibody TCX060 provided by the invention is obtained by modifying a cetuzumab monoclonal antibody as follows: (1) removing PEG modifications; (2) performing transforming into a humanized IgG1 subtype full-length antibody; and (3) inserting a flexible amino acid fragment between a CDR3 region and aCH2 region. The binding affinity of the TCX060 and humanized TNF alpha is close to that of cetuzumab, binding of the TNF alpha and a cell surface TNF receptor can be specifically blocked, the ADCC / CDCfunction of the TCX060 is close to that of the PEG cetuzumab monoclonal antibody, the immunogenicity of the TCX060 is obviously lower than that of the cetuzumab monoclonal antibody, the risk that theantibody generates immunogenicity in bodies is reduced while removing the ADCC / CDC function, and excellent application value can be achieved.
Owner:北京天成新脉生物技术有限公司
Polypeptide for preventing novel coronavirus pneumonia COVID-19 and application thereof
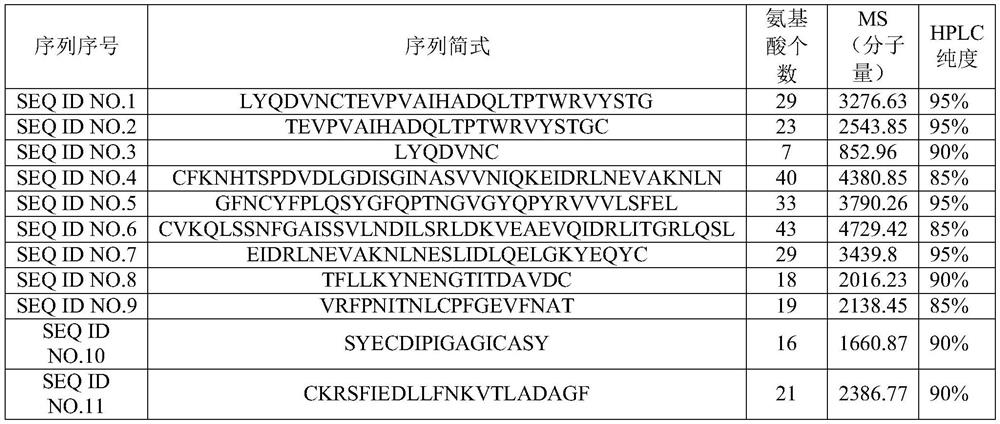
PendingCN114057852AGood antigenicityQuick filterSsRNA viruses positive-senseViral antigen ingredientsChemical synthesisVaccine antigen
The invention relates to the fields of immunological technology, biotechnology and biological medicine, in particular to polypeptide for preventing novel coronavirus pneumonia COVID-19 and application of the polypeptide. The amino acid sequence of the antigenic polypeptide is selected from any one of the following: 1) polypeptide with the amino acid sequence as shown in SEQ ID NO.1-SEQ ID NO.11; 2) polypeptide having 85% or more of the same amino acid sequence as the amino acid sequence as shown in SEQ ID NO.1-SEQ ID NO.11; and 3) polypeptide obtained by subjecting the amino acid sequences shown as SEQ ID NO.1-SEQ ID NO.11 to substitution and / or deletion and / or addition of one or more amino acid residues and having the same functions. The polypeptide for preventing novel coronavirus pneumonia COVID-19 provided by the invention is obtained by chemical synthesis of a specific antigenic fragment screened from a novel coronavirus S protein sequence, and an immunogen experiment proves that the polypeptide has relatively strong antigenicity, can induce a neutralizing antibody in a human body, and is expected to become a polypeptide vaccine antigen, and a new vaccine is provided for preventing novel coronavirus pneumonia COVID-19.
Owner:TSINGHUA UNIV
Novel pill for soothing liver and regulating qi
ActiveCN101433694BReduce the risk of side effectsReduce manufacturing costDigestive systemUnknown materialsMedicinal herbsPharmaceutical drug
The invention discloses an liver-soothing Qi-regulating pill, which consists of twenty-one medicinal materials such as Chinese gentian and rhizoma cyperi. The pill is characterized by removing talc on the basis of the prior preparation process and retaining the prior medicinal efficacy. The invention also discloses a preparation method and application of the novel liver-soothing Qi-regulating pill.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
Vaccine for preventing novel coronavirus pneumonia COVID-19 and preparation method thereof
PendingCN114053400ALow site requirementsSmall and simple molecular structureViral antigen ingredientsAntiviralsInfections problemsVaccine Production
The invention relates to the fields of immunological technology, biotechnology and biological medicine, in particular to a vaccine for preventing novel coronavirus pneumonia COVID-19 and a preparation method thereof. A composition of immunogenic conjugates includes at least two combinations of immunogenic conjugates containing the same carrier protein or at least two combinations of immunogenic conjugates containing two different carrier proteins. The composition of the immunogenic conjugates is used for preparing a vaccine for preventing COVID-19. Compared with a traditional attenuated vaccine, an inactivated vaccine and other novel vaccines, the vaccine can quickly cope with virus variation; the field requirement for vaccine production is reduced, and large-scale batch production is easy to realize; the research and development cost and the research and development period are reduced; safety, no toxicity and stability are realized; as the molecular structure is small and simple, the problems of serious complications and iatrogenic infection are rarely caused.
Owner:LIAONING CHENGDA BIOTECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com