Chimeric T cell receptor STAR and application thereof
A cell receptor and cell technology, applied in the field of treatment and diagnosis of diseases, antibody-T cell receptor chimeric receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1, construction of STAR targeting EGFR
[0101] The specific construction method is as follows:
[0102] 1. Determination of the sequence of the TCR constant region
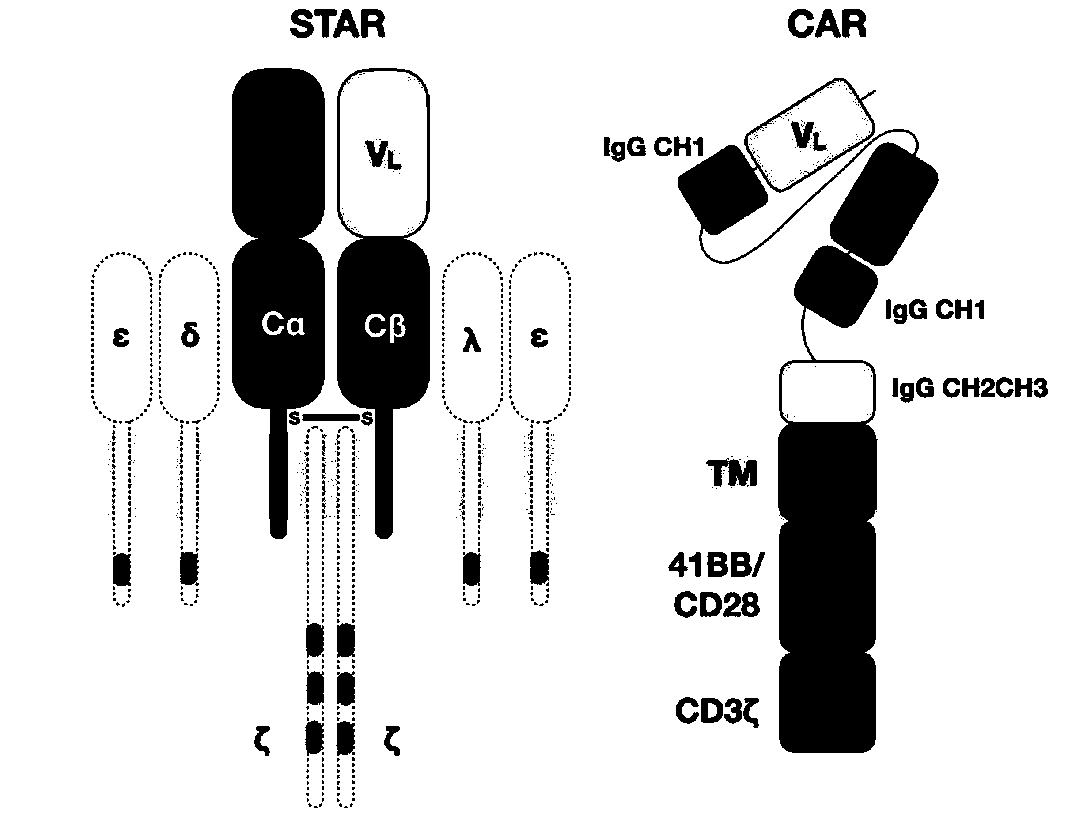
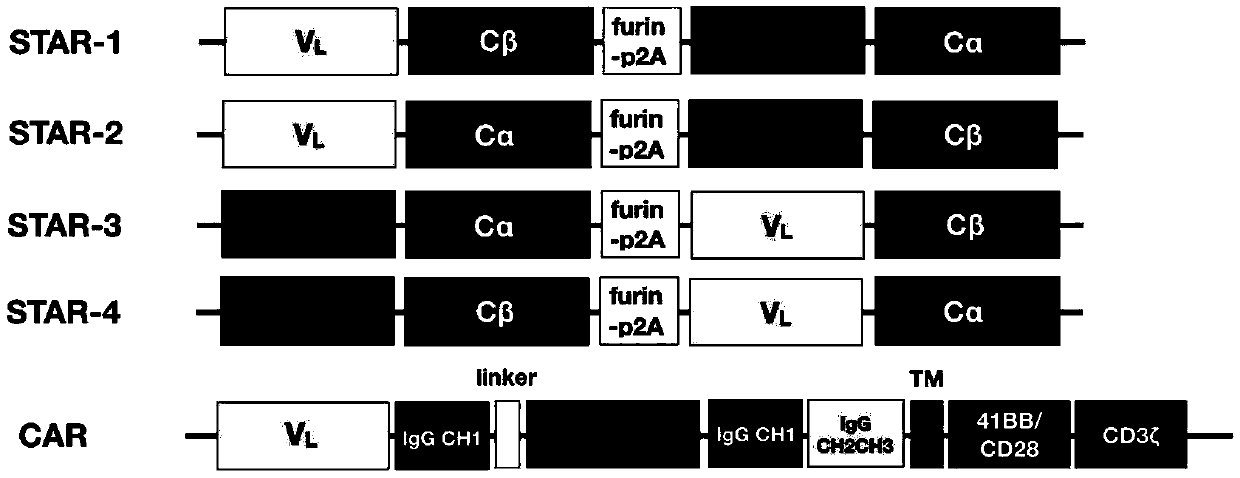
[0103] The constant regions (C regions) of the α chain and β chain of TCR in STAR are obtained from the cDNA of human peripheral blood T cells by PCR molecular cloning; on the basis of the original TCR sequence, the α chain and β chain are separately The 48th and 57th amino acid positions of the constant region were mutated to cysteine to help form an additional disulfide bond between the α chain and the β chain, increasing their mutual pairing efficiency, and named it E1-TCR.
[0104] 2. Determination of the antibody sequence targeting EGFR
[0105] The antibody heavy chain variable region (VH) and the antibody light chain variable region (VL) select cetuximab (Cetuximab, referred to as Cetux), only as an example to explain the content of the present invention, and other known antibodies can b...
Embodiment 2
[0119] Example 2. Functional verification of STAR targeting EGFR
[0120] 1. Lentiviral Packaging
[0121] The pHAGE vector carrying the target gene and the packaging plasmids pMD2.G and psPAX2 were transfected into 293T cells in proportion (using PEI transfection). Collect the supernatant of the cell culture medium at 48 hours and 72 hours, mix the supernatant with PEG8000, let it stand overnight and then centrifuge to obtain the virus pellet. Resuspend with a small volume of medium to achieve the effect of virus concentration.
[0122] 2. Lentiviral Infection of Human T Cell Lines
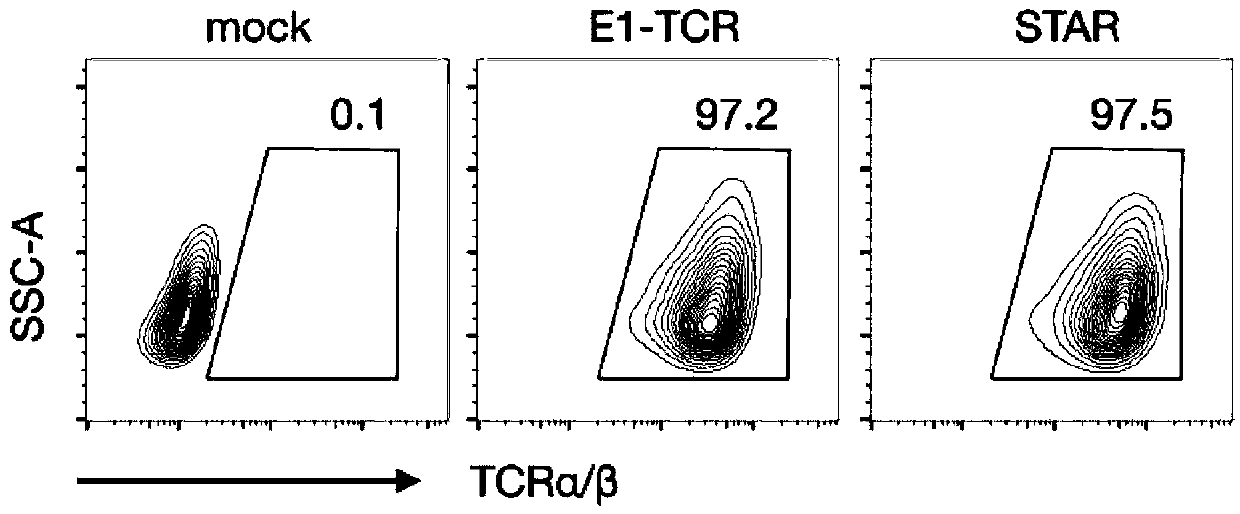
[0123] Infect Jurkat clone 5 cells (Jurkat subclone with endogenous TCR deletion) with lentivirus carrying the target gene. The concentrated lentivirus and the transfer agent Polybrene were added to the T cell culture medium, and centrifuged at 1500rpm at 32°C for 2 hours. After 3 days of infection, the fluorescent reporter gene can be observed and the expression of the target protein can be ...
Embodiment 3
[0133] Example 3, construction of STAR targeting CD19
[0134] The specific construction method is as follows:
[0135] 1. Determination of the sequence of the TCR constant region
[0136] The cDNA derived from human peripheral blood T cells or mouse spleen T cells is obtained by PCR molecular cloning; on the basis of the original TCR sequence, the 48th and 57th amino acid sites of the constant regions of the α chain and β chain are mutated, respectively It is cysteine to help form an additional disulfide bond between the α chain and the β chain to increase their mutual pairing efficiency, and they are named E1-TCR (human source) or E11-TCR (mouse source) respectively.
[0137] 2. Determination of the antibody sequence targeting CD19
[0138] Antibody heavy chain variable region (VH) and antibody light chain variable region (VL) select the scFv fragment of CD19-specific mouse monoclonal antibody (clone number FMC63), only as an example to explain the content of the present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com