Polypeptide for preventing novel coronavirus pneumonia COVID-19, immunogenic conjugate and application thereof
A COVID-19, coronavirus technology, applied in the fields of biotechnology, biomedicine, and immunology, can solve problems such as the loss of efficacy of biohazardous original vaccines, achieve easy mass production, reduce site requirements, and reduce antigenicity effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthetic method of the target polypeptide (the amino acid sequence is the polypeptide shown in SEQ ID NO.1).
[0040] 1. The polypeptide whose amino acid sequence is shown in SEQ ID NO.1 was prepared by using the conventional method of solid-phase polypeptide synthesis, and the specific steps are as follows.
[0041] (1) Remove Fmoc protection.
[0042] Fill a commercially available Rink Amide AM resin into an organic-solvent-resistant reaction tube with a filter and cap tightly with an organic-solvent-resistant cap. After washing with DMF (dimethylformamide) for 1 minute, add an excess of 20% piperidine / DMF (volume ratio) solution, cap tightly and shake the reaction tube gently to mix it evenly and keep the Fmoc protection removed for 15 minutes . After draining, wash three times with DMF.
[0043] (2) Peptide bond condensation.
[0044] The Fmoc-protected amino acid of 3 times of resin amino molar weight, the activator HBTU of 3 times of resin amino mol...
Embodiment 2
[0053] Example 2 Preparation method of the synthetic peptide immunogenic conjugate shown in SEQ ID NO.1.
[0054] The synthetic peptides prepared in Example 1 were combined with CRM197, TT, DT, and bacterial outer membrane proteins to form conjugated polypeptides through Linker.
[0055] TT tetanus toxin protein and tetanus toxin carrier protein TT can be obtained from Bacillus tetani through fermentation, lysis, centrifugation and chromatographic purification.
[0056] DT diphtheria toxin protein and diphtheria toxin carrier protein DT can be obtained from diphtheria bacillus through fermentation, cracking, centrifugation and chromatography purification.
[0057] CRM197 Recombinant Diphtheria Toxin Protein, CRM197 is a diphtheria bacillus reconstructed from the gene sequence, which is obtained through fermentation, lysis, centrifugation, and chromatography purification.
[0058] Meningococcal outer membrane protein: obtained from meningococcus through fermentation, lysis, ce...
Embodiment 3
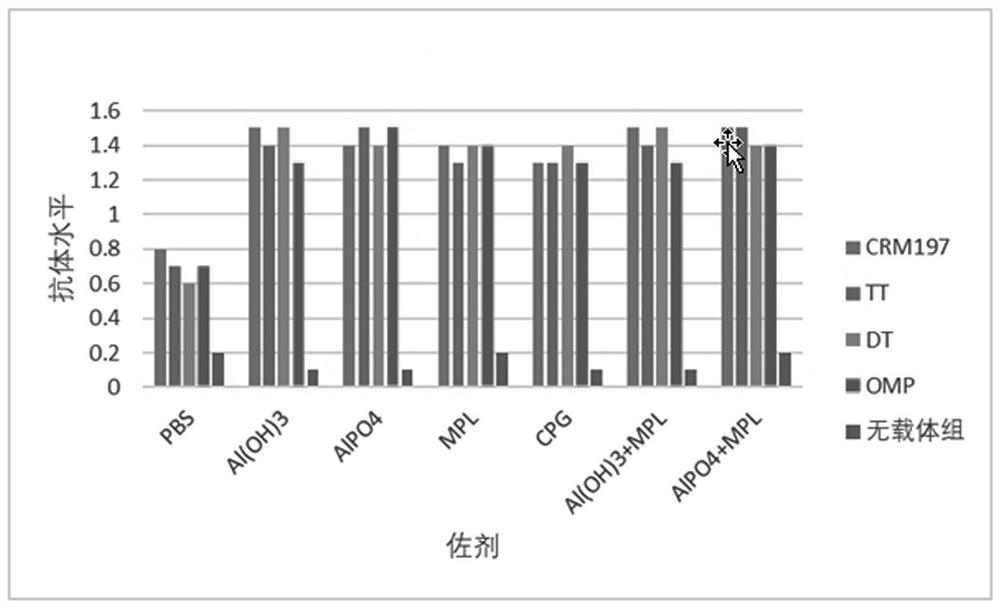
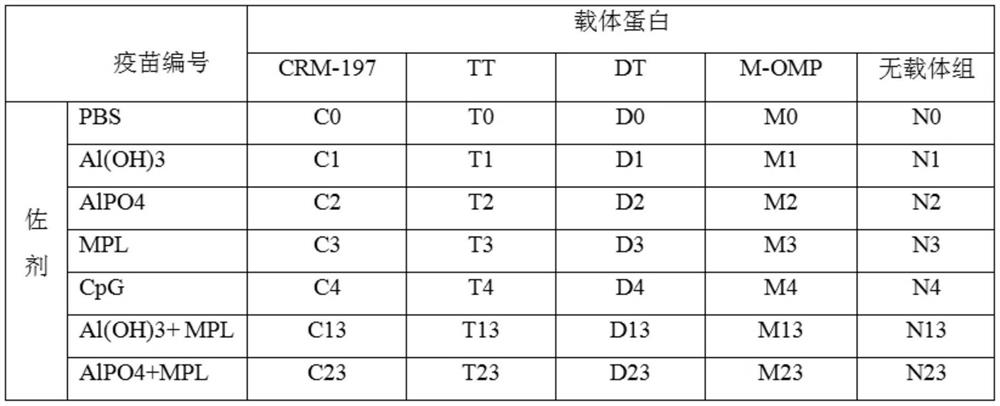
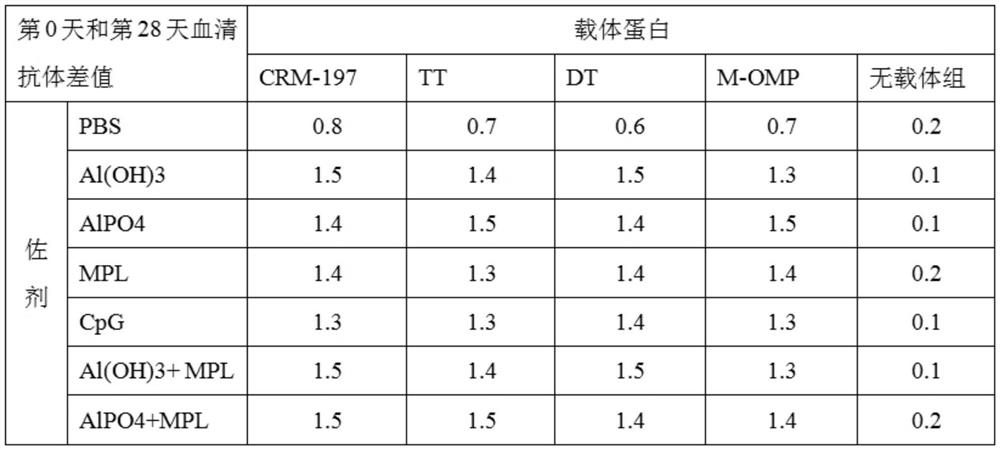
[0071] Example 3 Preparation of vaccine and immune effect.
[0072] 1. Vaccine preparation.
[0073] 1. Materials.
[0074] Polypeptide protein: the amino acid sequence is the polypeptide shown in SEQ ID NO.1.
[0075] Carrier protein: diphtheria toxin null mutant (CRM197), tetanus toxoid (TT), diphtheria toxoid (DT), meningococcus outer membrane protein (Meningococcus OMP).
[0076] Adjuvants: aluminum hydroxide (Al(OH)3), aluminum phosphate (AlPO4), MPL, CpG.
[0077] 2. Preparation method.
[0078] Take the immunogenic conjugate, mix it with adjuvant and PBS solution, shake it at room temperature for 1 hour, 30RPM, the resulting final concentration contains 100μg / ml polypeptide antigen and 0.5mg / ml adjuvant, which is the final sample; or first mix the immunogen Adsorb the conjugate to the aluminum adjuvant, shake at room temperature for 1 hour, 30RPM, then add MPL or CpG adjuvant, shake at room temperature for 1 hour, 30RPM, the resulting final concentration contains 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com