Polypeptide for preventing novel coronavirus pneumonia COVID-19, immunogenic conjugate and application thereof
A COVID-19 and coronavirus technology, applied in the fields of biotechnology, biomedicine, and immunology technology, can solve the problems of loss of efficacy of biohazardous original vaccines, etc., and achieve easy large-scale mass production, reduce site requirements, and small molecular structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 The synthetic method of the target polypeptide (the amino acid sequence is the polypeptide shown in SEQ ID NO. 1).
[0040] 1. A polypeptide whose amino acid sequence is shown in SEQ ID NO. 1 is prepared by using a conventional solid-phase method for synthesizing polypeptides, and the specific steps are as follows.
[0041] (1) De-Fmoc protection.
[0042] Commercially available Rink Amide AM resin was placed in a filter, organic solvent-resistant reaction tube and tightly capped with an organic solvent-resistant cap. After washing with DMF (dimethylformamide) for 1 minute, an excess of 20% piperidine / DMF (volume ratio) solution was added, and the reaction tube was gently shaken after capping to make it evenly mixed and kept to remove Fmoc protection for 15 minutes. . After draining, it was washed three times with DMF.
[0043] (2) Peptide bond condensation.
[0044] Fmoc-protected amino acids with 3 times the molar amount of resin amino groups, 3 times th...
Embodiment 2
[0053] Example 2 The preparation method of the synthetic peptide immunogenic conjugate shown in SEQ ID NO.1.
[0054] The synthetic peptides prepared in Example 1 were combined with CRM197, TT, DT, and bacterial outer membrane proteins to form co-conjugated polypeptides through Linker.
[0055] TT tetanus toxin protein and tetanus toxin carrier protein TT can be obtained by tetanus bacillus through fermentation, lysis, centrifugation and chromatography purification.
[0056] DT diphtheria toxin protein and diphtheria toxin carrier protein DT can be obtained by Bacillus diphtheriae through fermentation, lysis, centrifugation and chromatography purification.
[0057] CRM197 recombinant diphtheria toxin protein, CRM197 is a diphtheria bacillus with reconstructed gene sequence, obtained by fermentation, lysis, centrifugation and chromatography purification.
[0058] Meningococcal outer membrane protein: obtained by fermentation, lysis, centrifugation and chromatography purificati...
Embodiment 3
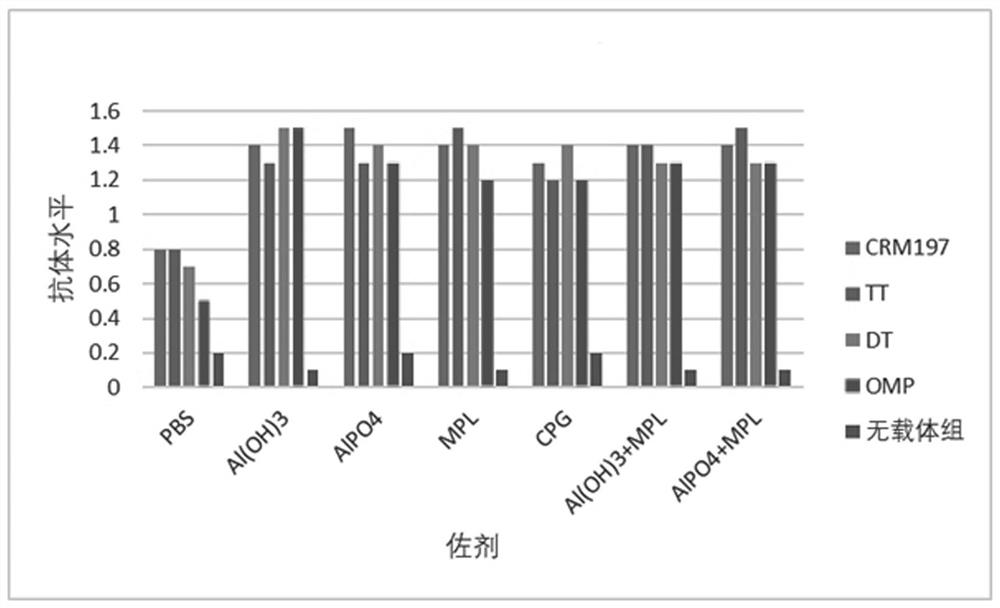
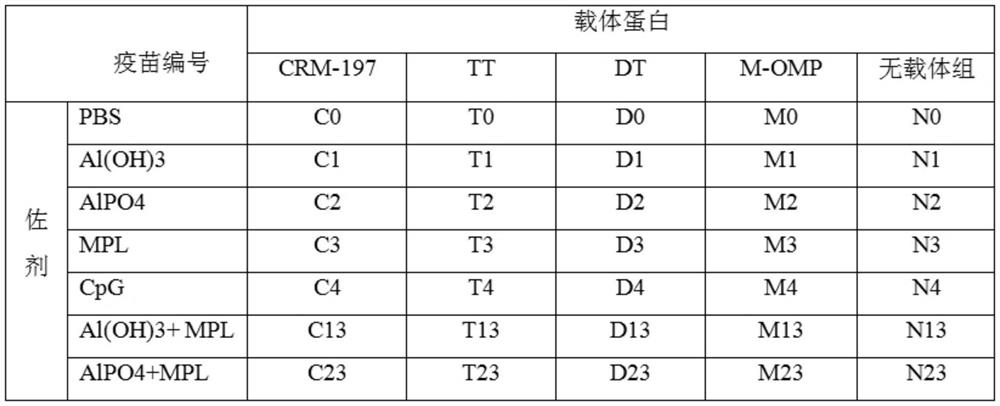
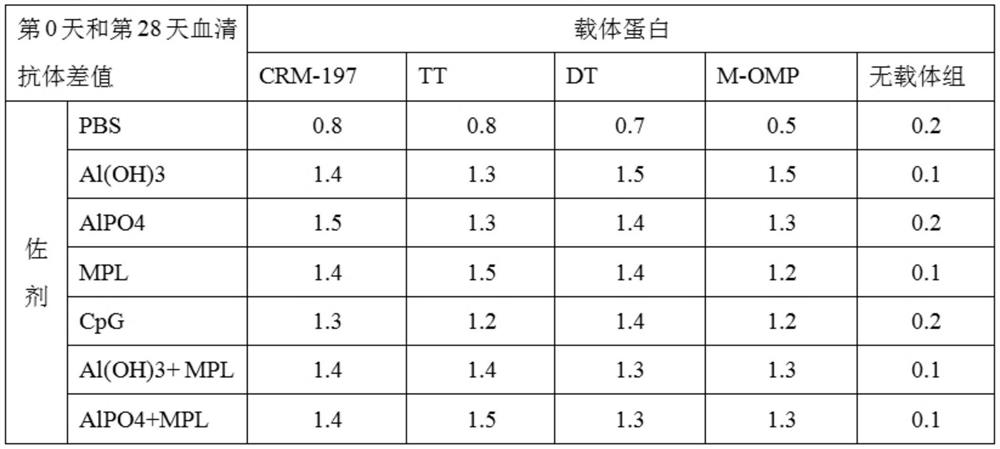
[0071] Example 3 Preparation of vaccine and immune effect.
[0072] 1. Vaccine preparation.
[0073] 1. Materials.
[0074] Polypeptide protein: the amino acid sequence is the polypeptide shown in SEQ ID NO.1.
[0075] Carrier proteins: Diphtheria toxin null mutant (CRM197), Tetanus toxoid (TT), Diphtheria toxoid (DT), Meningococcus OMP.
[0076] Adjuvants: aluminum hydroxide (Al(OH)3), aluminum phosphate (AlPO4), MPL, CpG.
[0077] 2. Preparation method.
[0078] Mix the immunogenic conjugate with adjuvant and PBS solution, shake at room temperature for 1 hour, 30 RPM, the final concentration of which contains polypeptide antigen 100 μg / ml and adjuvant 0.5 mg / ml is the final sample; The sexual conjugate was adsorbed on aluminum adjuvant, shaken at room temperature for 1 hour, 30RPM, then added MPL or CpG adjuvant, shaken at room temperature for 1 hour, 30RPM, the final concentration contained polypeptide antigen 100μg / ml, aluminum adjuvant 0.5mg / ml, MPL or CpG content o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com