Anti-TNF-α humanized monoclonal antibody tcx060 with low immunogenicity and low ADCC/CDC function and its application
A monoclonal antibody, humanized technology, applied in the direction of DNA/RNA fragments, antibodies, applications, etc., can solve the problems of reducing the therapeutic benefit of patients, increasing the risk of side effects, limiting the re-administration of antibodies, etc., to achieve great application potential and Value, low immunogenicity, effect of improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
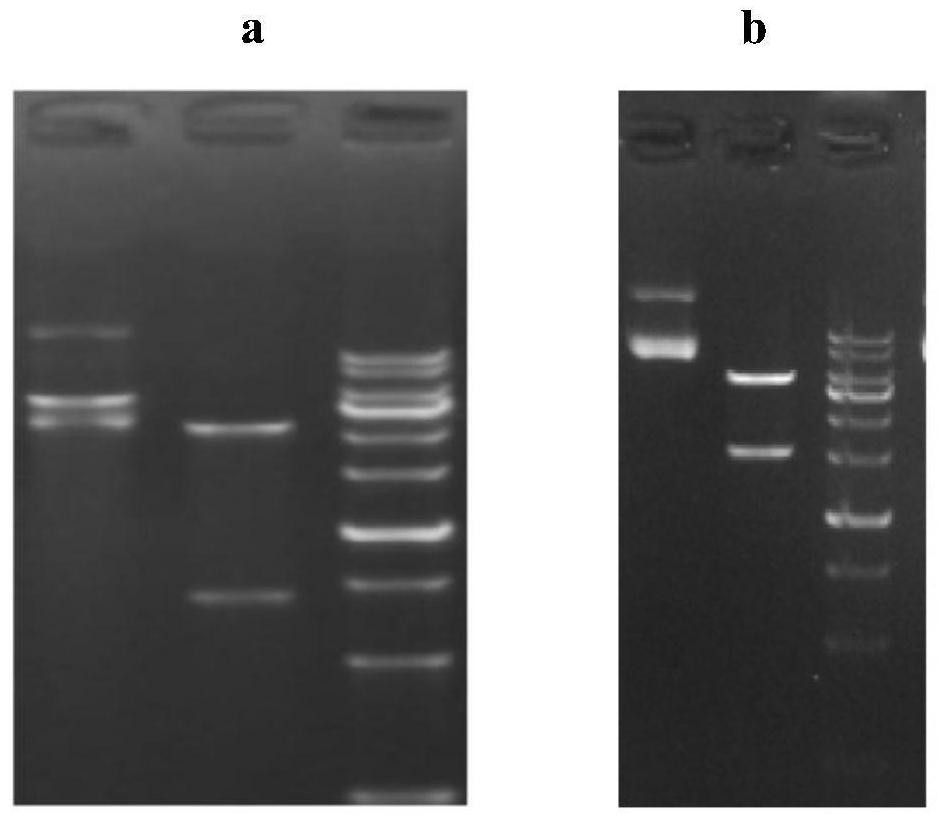
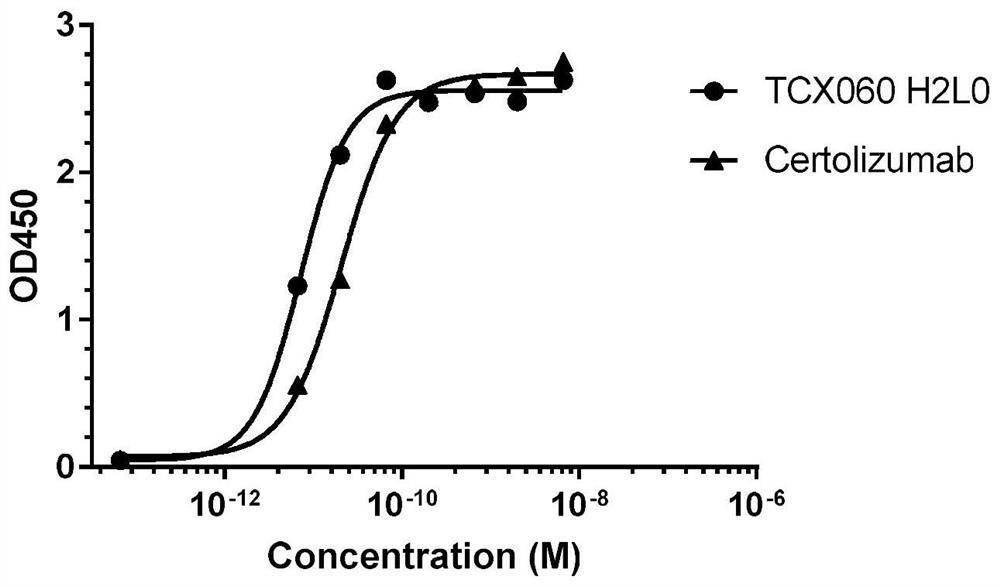
[0053] Example 1 Analysis and Design of Certolizumab Monoclonal Antibody with Low ADCC / CDC Function and Reduced Immunogenicity
[0054] The present invention analyzes and evaluates the original sequence of the certoliz monoclonal antibody by using the commercial DNAStarTM software, and the analysis result shows that the immunogenicity of the original certoliz monoclonal antibody sequence is very low. It is speculated that after the PEGylation of the original certolizumab monoclonal antibody was performed to remove the ADCC / CDC function, the multimer of the antibody increased, which in turn caused the ADA of the drug to be as high as 23%. Therefore, it is necessary to remove the PEGylation modification of the original certolizumab monoclonal antibody, and at the same time reduce its ADCC / CDC function. The amino acid sequences of the full length of the light chain and the variable region of the heavy chain of the original certo beads are shown in SEQ ID NO.1 and SEQ ID NO.2, res...
Embodiment 2
[0056] Example 2 Construction of the expression vector of anti-TNF-α humanized monoclonal antibody TCX060
[0057] According to the amino acid sequence of the full-length heavy chain and light chain of the anti-TNF-α fully humanized monoclonal antibody H2L0 obtained in Example 1 and the codon preference of the host, the nucleotides encoding the heavy chain H2 and the light chain L0 were designed sequence (wherein the nucleotide sequence of L0 is shown in SEQ ID NO.6, and the nucleotide sequence of H2 is shown in SEQ ID NO.5), and the enzyme cutting sites on both sides of the light chain sequence are designed to be Hind III+EcoR I. Design the enzyme cutting sites on both sides of the heavy chain sequence as Hind III+EcoR I, send the full-length heavy chain and light chain full-length sequences carrying the enzyme cutting sites to Jinweizhi Company to synthesize the whole gene sequence, and synthesize the used The connection vector is pUC57. Using pEE12.4 (for heavy chain expre...
Embodiment 3
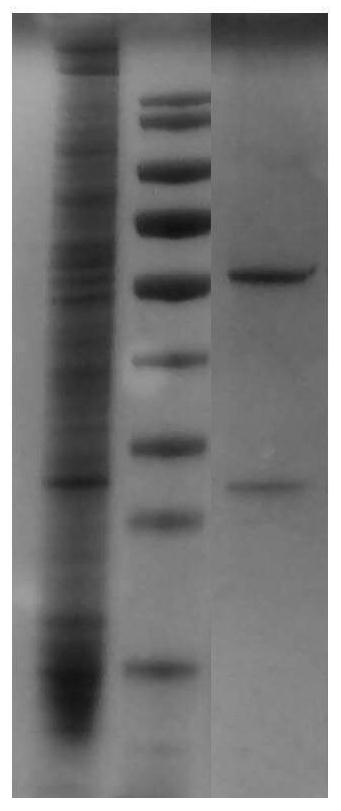
[0059] Example 3 Transient Expression of Anti-TNF-α Humanized Monoclonal Antibody TCX060
[0060] The Escherichia coli DH5α strains obtained in Example 2 carrying the expression vectors of the full-length genes of the heavy chain H2, H0 and light chain L0 were cultured, the cultures were harvested, and the Qiagen UltraPure plasmid DNA purification kit was used to extract and purify the heavy chain and light chain full-length gene expression vector. The above-mentioned purified plasmid DNA was transfected into 293F cells using the liposome method kit of Invitrogen Company, and the transfection method was referred to the kit instructions.
[0061] The positive cells obtained by transfection were used for antibody expression, and the expression level of the monoclonal antibody TCX060 whose combination of heavy chain and light chain is H2L0 is shown in Table 2.
[0062] Table 2 The expression level detection result of monoclonal antibody TCX060 (mg / L)
[0063]
[0064]The ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com