Preparation method of stable dezocine injection
A technology of xin injection and dezocine, which is applied in the field of pharmaceutical preparations and can solve problems such as the difference in production amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
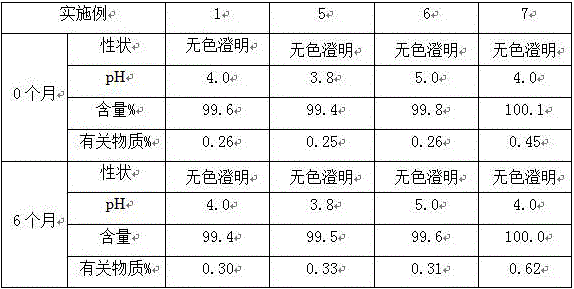
Examples
Embodiment 1
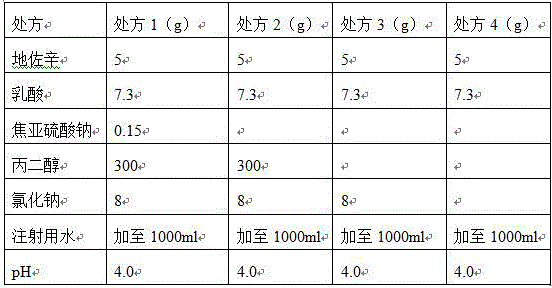
[0041] Prescription (1000 sticks)
[0042] Dezocine 5g
[0043] Lactic acid 7.3g
[0044] Add water for injection to 1000ml
[0045] pH 4.0
[0046] Preparation method: Add lactic acid to water for injection (temperature 50°C) with 70% of the prescription amount, saturate with nitrogen, and stir evenly. Add API and stir until completely dissolved. Adjust pH to 4.0 with 1mol / L sodium hydroxide solution, add water for injection to 1000ml; add 0.05% medicinal charcoal, supplement nitrogen, stir for 15 minutes, keep the temperature at 50 °C for about 30 minutes, and sterilize by coarse filtration Filtration, the obtained liquid is potted (with nitrogen gas, 1ml / piece), melted and sealed, and the finished product is obtained. After potting, the sample is put into a sterilization cabinet. Light inspection, labeling and packaging to get the finished product.
Embodiment 2
[0048] Prescription (1000 sticks)
[0049] Dezocine 5g
[0050] Lactic acid 2.5g
[0051] Add water for injection to 1000ml
[0052] pH 4.0
[0053] Preparation method: Add lactic acid to water for injection (temperature 40°C) with 70% of the prescription amount, saturate with nitrogen, and stir evenly. Add API and stir until completely dissolved. Adjust pH to 4.0 with 1mol / L sodium hydroxide solution, add water for injection to 1000ml; add 0.2% medicinal charcoal, supplement nitrogen, stir for 15 minutes, keep the temperature at 35~50°C for about 30 minutes, pass through coarse filtration, After sterilization and filtration, the obtained liquid is potted (with nitrogen gas, 1ml / piece), melted and sealed, and the finished product is obtained. After potting, the sample is put into a sterilization cabinet. Light inspection, labeling and packaging to get the finished product.
Embodiment 3
[0055] Prescription (1000 sticks)
[0056] Dezocine 5g
[0057] Lactic acid 25g
[0058] Add water for injection to 1000ml
[0059] pH 4.0
[0060]Preparation method: Add lactic acid to 70% water for injection (temperature 45°C) of the prescription, saturate with nitrogen, and stir evenly. Add raw material drug and stir until completely dissolved. Use 1mol / L sodium hydroxide solution to adjust the pH to 4.0, add water for injection to 1000ml; add 0.05% medicinal charcoal, supplement nitrogen, stir for 15 minutes, keep the temperature at 45°C for about 30 minutes, and then pass coarse filtration to sterilize After filtering, the obtained medicinal solution is potted (nitrogen gas, 1ml / bottle), melted and sealed, and finally the finished product is obtained. After the potting is completed, the sample is put into a sterilizing cabinet, and the sterilization condition is: 121°C for 15 minutes. Light inspection, labeling and packaging to get the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com