Environmental pH stimuli-responsive type tumor targeting and controlled drug release nano-carrier and preparation method of nano-carrier
A tumor-targeting, stimuli-responsive technology, applied in the field of biopolymer materials and nanometers, can solve the problems of high cytotoxicity, nucleic acid is easy to be hydrolyzed, and cannot distinguish cancer cells from normal cells, etc., so as to prolong the circulation time and not easily release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] [Example 1] Preparation of dendritic nano-prodrugs
[0033] (1) Amphiphilic block copolymer (PBLA-b-PEG-OCH 3 ) Synthesis: The copolymer of polybenzyl aspartate and PEG was synthesized by NCA method, and N-carboxy-L-aspartic acid was prepared by reacting benzyl L-aspartate (BLA) with trimer phosgene -Benzyl ester-cyclic anhydride (BLA-NCA). Using methoxypolyethylene glycol amine (MPEG-NH2) as an initiator to initiate ring-opening polymerization of NCA, poly(ethylene glycol)-poly(benzyl aspartate) amphiphilic block copolymers with different molecular weights were synthesized.
[0034] Dissolve 80 mg-100 mg BLA-NCA in 6 mL of anhydrous DMF solution at room temperature. Subsequently, 100mg-200mg of methoxyl PEG-NH 2 (MPEG-NH 2 ) (or MAL-PEG-NH 2 ) was added to the solution. Pour in 0 O The precipitate was separated in diethyl ether of C. The precipitate was collected by filtration and washed. Finally dry under vacuum.
[0035] (2) A multifunctional tumor-targetin...
Embodiment 2
[0041] [Example 2] Preparation of Hyperbranched Nanomicelles
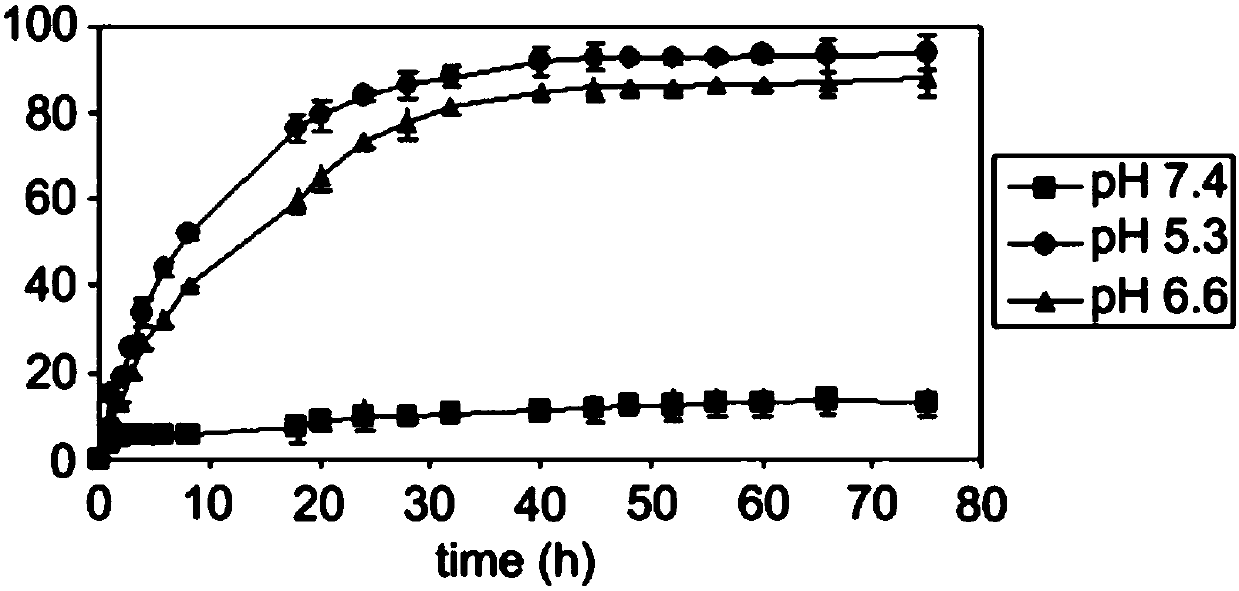
[0042] The polymer prodrug obtained in Example 1 can be self-assembled in an aqueous medium to form nanomicelles with a core-shell structure. After entering the target tumor tissue, due to the pH sensitivity of the hydrazone bond, the hydrazone bond is in the It can be broken in an acidic environment, but will not break in the pH environment of normal tissues, so as to realize the intelligent controlled release of drugs.
[0043] PAMAM-P(LA-HYD-DOX)-b-PEG-OCH 3 / F3 copolymer dissolved in DI water. Dialysis for 96 hours followed by lyophilization yielded PAMAM-P(LA-Hyd-DOX)-b-PEG-OCH 3 / F3 micelles.
Embodiment 3
[0044] [Example 3] Preparation and Characterization of DOX-loaded Polymer Prodrug
[0045] Preparation of amphiphilic polymer prodrug PAMAM–P (LA-Hyd -DOX)-b-PEG-OCH using PAMAM as a macromolecule carrier 3 / F3, and carry out nuclear magnetic resonance on the reaction drug substance and the reaction product of each step ( 1 HNMR) characterization; (such as figure 1 ), 1 H NMR (400MHz, DMSO) δ 7.94 (s, 1H), 5.33 (s, 1H), hydrogen 3.44 (d, J = 59.2 Hz,20H), attributed to the peak 1.05 of PEG (d, J = 151.7 Hz, 1H). Hydrogen on PAMAM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com