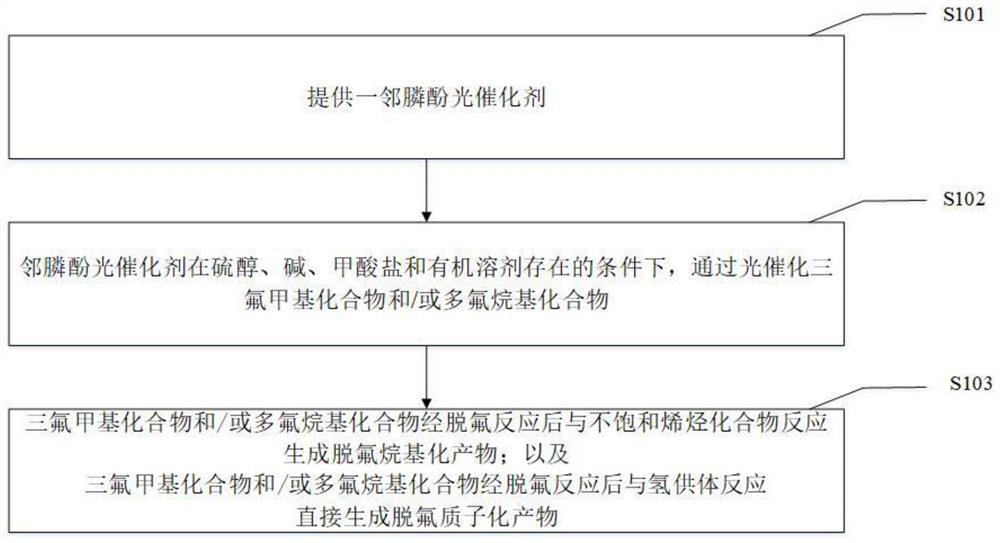
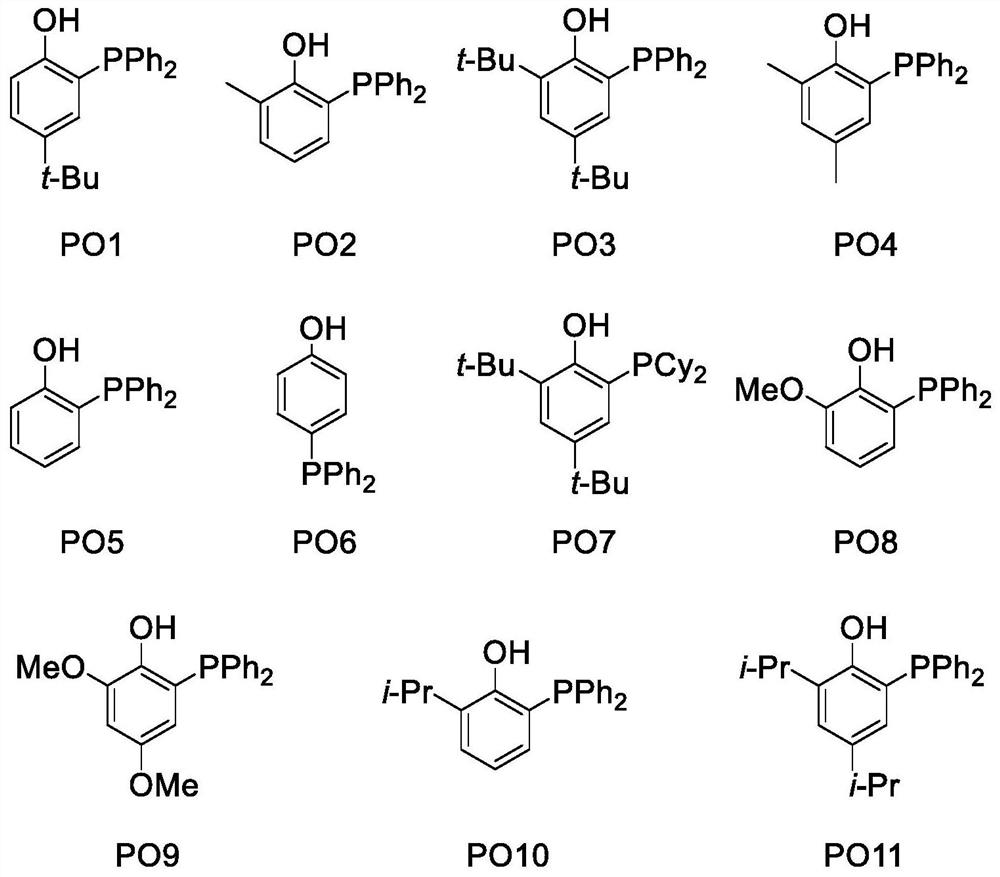
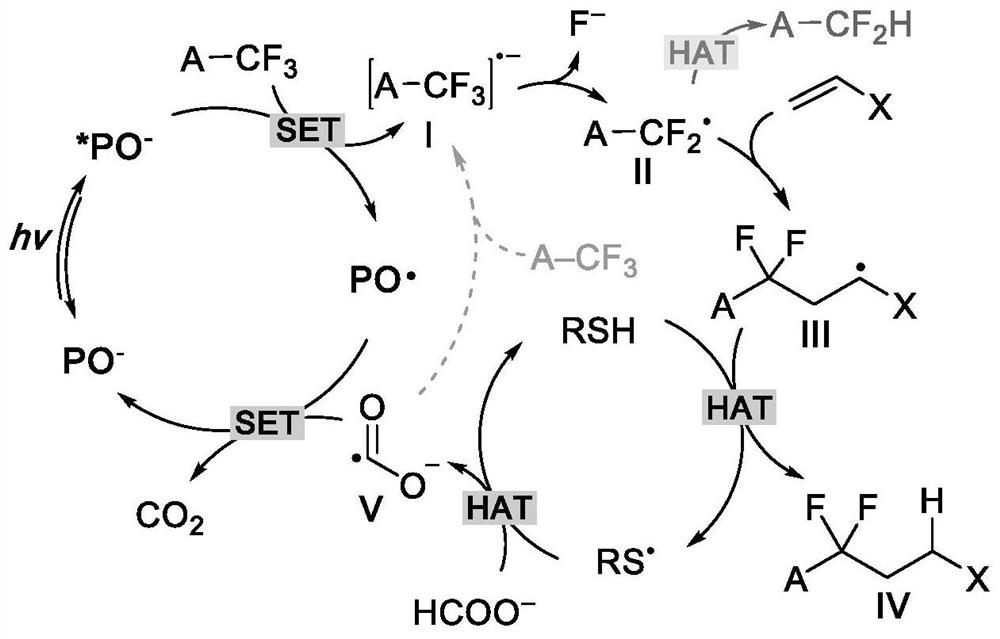
Method for defluorination alkylation and defluorination protonation reaction by using o-phosphinophenol photocatalyst
A photocatalyst and defluorinated alkyl technology, applied in catalytic reaction, organic chemical method, cyanide reaction preparation, etc., can solve the problems of high cost, environmental pollution, etc., and achieve the effects of prolonging life, simplifying treatment methods, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1, preparation of 2,2-difluoro-6-hydroxyl-N-phenylhexanamide
[0097]
[0098] Following general procedure A, the yields are shown in Table 2.
[0099] The 2,2-difluoro-6-hydroxyl-N-phenylhexanamide obtained in Example 1 was carried out by nuclear magnetic resonance 1 H NMR, 13 C NMR and 19 F NMR analysis.
[0100] Figure 4 is 2,2-difluoro-6-hydroxy-N-phenylhexanamide 1 H NMR spectrum.
[0101] like Figure 4 as shown, 1 H NMR (400MHz, CDCl 3 )δ8.09(s,1H),7.57(d,J=7.9Hz,2H),7.37(t,J=7.9Hz,2H),7.19(t,J=7.4Hz,1H),3.66(t, J=5.7Hz, 2H), 2.32–2.12(m, 2H), 1.73(s, 1H), 1.62(dd, J=7.0, 4.0Hz, 4H).
[0102] Figure 5 is 2,2-difluoro-6-hydroxy-N-phenylhexanamide 13 C NMR spectrum.
[0103] like Figure 5 as shown, 13 C NMR (101MHz, CDCl 3 )δ162.2(t, J=28.6Hz), 136.0, 129.2, 125.6, 120.3, 118.3(t, J=253.6Hz), 62.2, 33.5(t, J=23.2Hz), 31.9, 18.1(t, J = 4.5Hz).
[0104] Image 6 is 2,2-difluoro-6-hydroxy-N-phenylhexanamide 19 F NMR spectrum.
[0105...
Embodiment 2
[0106] Embodiment 2, preparation 2,2-difluoro-6-hydroxyl-N-(4-methoxyphenyl) hexanamide
[0107]
[0108] Following general procedure A, the yields are shown in Table 2.
[0109] The 2,2-difluoro-6-hydroxyl-N-(4-methoxyphenyl) hexanamide obtained in embodiment 2 was carried out by nuclear magnetic resonance 1 H NMR, 13 C NMR and 19 F NMR analysis obtains the result:
[0110] 1 H NMR (400MHz, DMSO) δ10.38(s, 1H), 7.59(d, J=9.1Hz, 2H), 6.93(d, J=9.1Hz, 2H), 3.74(s, 3H), 3.41(t ,J=5.7Hz,2H),2.25–2.04(m,2H),1.52–1.42(m,4H).
[0111] 13 C NMR (101MHz, DMSO) δ162.3(t, J=29.4Hz), 156.8, 130.7, 122.9, 118.7(t, J=251.5Hz), 114.3, 60.7, 55.7, 34.1(t, J=23.3Hz) ,32.2,18.6(t,J=4.3Hz).
[0112] 19 F NMR (376MHz, DMSO) δ-104.1 (2F, t, J=17.3Hz).
Embodiment 3
[0113] Example 3, preparation of 2,2-difluoro-6-hydroxyl-N-(pyridin-3-yl)hexanamide
[0114]
[0115] Following general procedure A, the yields are shown in Table 2.
[0116] The 2,2-difluoro-6-hydroxyl-N-(pyridin-3-yl) hexanamide obtained in embodiment 3 was carried out by nuclear magnetic resonance 1 H NMR, 13 C NMR and 19 F NMR analysis obtains the result:
[0117] 1 H NMR (400MHz, CDCl 3 )δ8.69(s,1H),8.47(s,1H),8.43(d,J=4.4Hz,1H),8.19(d,J=8.4Hz,1H),7.34(dd,J=8.4,4.7 Hz,1H),3.68(t,J=5.7Hz,2H),2.31–2.15(m,2H),1.95(s,1H),1.68–1.60(m,4H).
[0118] 13 C NMR (101MHz, CDCl 3 )δ162.8(t, J=29.5Hz), 146.4, 141.6, 133.3, 127.9, 123.9, 118.1(t, J=253.4Hz), 62.1, 33.5(t, J=23.1Hz), 31.9, 18.1(t ,J=4.4Hz).
[0119] 19 F NMR (376MHz, CDCl 3 )δ-105.2 (2F,td,J=17.3,2.5Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com