Catalyst for Michael addition reaction and preparation method of nitro fatty aldehyde
A catalyst, aliphatic aldehyde technology, applied in the preparation of organic compounds, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve the problems of high cost, many synthesis steps, unsuitable for large-scale industrial production, etc. Enantiomeric and enantioselective, effect of accelerating reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
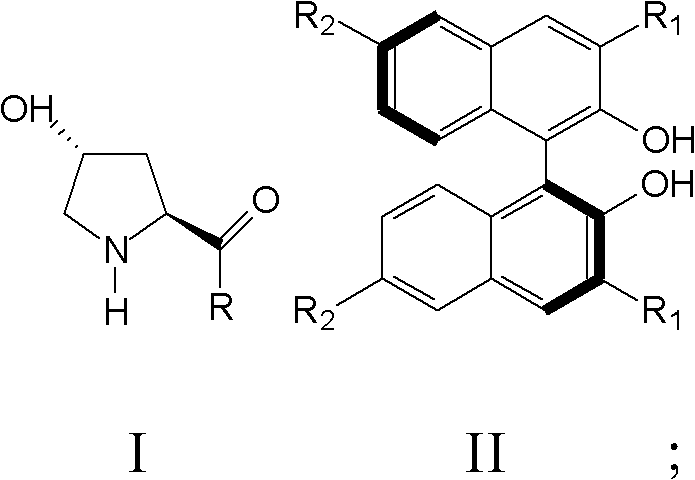
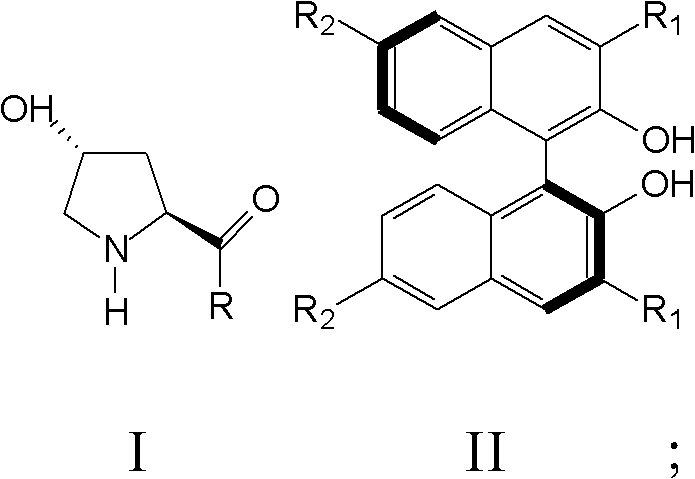
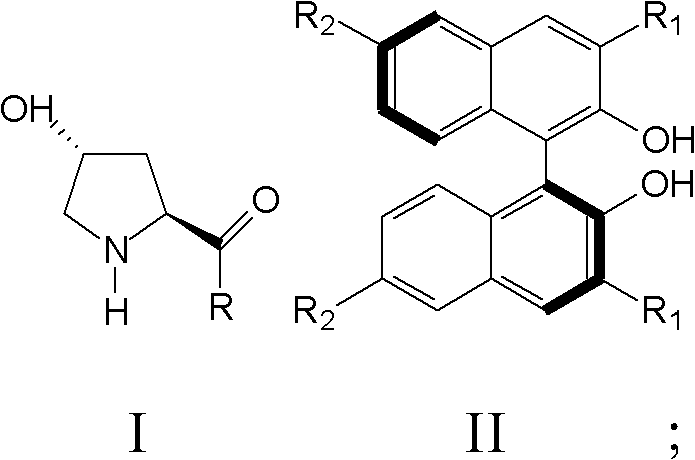
[0040]The present invention also provides a preparation method of nitro fatty aldehyde, comprising: a) mixing a compound represented by formula I and a compound represented by formula II in a solvent to obtain a mixed solution;
[0041] Wherein, R is a dialkylamine group or a cycloalkylamine group; R 1 and R 3 They are hydrogen, bromine, iodine, phenyl, 2,4,6-trimethylphenyl, trimethylsilyl or triphenylsilane, respectively.
[0042] b) Mix fatty aldehyde and nitroalkene in the mixed solution and react at 0°C until the nitroalkene completely disappears;
[0043] c) Quenching the reaction with hydrochloric acid, extracting the reaction product, drying and separating by chromatography to obtain nitro fatty aldehyde.
[0044] According to the present invention, the reaction substrates are aliphatic aldehydes and nitroalkenes, and the nitroalkenes are preferably aromatic nitroalkenes or aliphatic nitroalkenes, more preferably benzene, furan, thiophene and derivatives thereof with...
Embodiment 1
[0058] Mix 0.33 g, 1.3 mmol of BOP-Cl with 0.3 g, 1.3 mmol of N-tert-butoxycarbonyl-trans-4-hydroxy-L-proline and 0.3 mL, 2.2 mmol of triethylamine in 3 mL of dichloromethane mixed in a reaction flask to obtain a suspension, and the resulting suspension was stirred for 30 minutes under an ice bath, and then 1.3mmol of hexahydropyridine and 0.15mL, 1.1mmol of triethylamine were mixed in 1mL of dichloromethane, and dropped at the same temperature into the reaction bottle. After further stirring for 16 hours, the reaction temperature was slowly raised from 0°C to room temperature, the reaction solution was filtered, the filtrate was diluted with 10 mL of dichloromethane, washed with saturated aqueous sodium bicarbonate (5 mL×2) and brine (5 mL×2), The aqueous phase was further extracted with 10 mL of dichloromethane, combined with the organic phase, dried, filtered and concentrated with anhydrous sodium sulfate to obtain the intermediate N-Boc-proline derivative, the compound sho...
Embodiment 2~10
[0063] According to Table 1 R 3 , R 4 The listed substituents select the reaction raw materials, use the method of Example 1 to prepare nitro fatty aldehyde, the yield of the obtained product is 92%-96%, and the ratio of diastereomers is greater than 95 / 5, even greater than 99 / 1 , the enantiomeric excess value is greater than 95%, even greater than 99%, these performances can all show that the catalyst provided by the present invention has higher catalytic efficiency, and the prepared nitro fatty aldehyde has better performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com